Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks
- PMID: 11209063
- PMCID: PMC14646
- DOI: 10.1073/pnas.98.2.670
Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks
Abstract
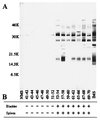
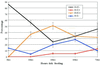
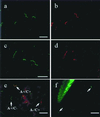
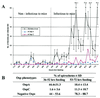
The genome of Borrelia burgdorferi encodes a large number of lipoproteins, many of which are expressed only at certain stages of the spirochete's life cycle. In the current study we describe the B. burgdorferi population structure with respect to the production of two lipoproteins [outer surface protein A (OspA) and outer surface protein C (OspC)] during transmission from the tick vector to the mammalian host. Before the blood meal, the bacteria in the tick were a homogeneous population that mainly produced OspA only. During the blood meal, the population became more heterogeneous; many bacteria produced both OspA and OspC, whereas others produced only a single Osp and a few produced neither Osp. From the heterogeneous spirochetal population in the gut, a subset depleted of OspA entered the salivary glands and stably infected the host at time points >53 hr into the blood meal. We also examined genetic heterogeneity at the B. burgdorferi vlsE locus before and during the blood meal. In unfed ticks, the vlsE locus was stable and one predominant and two minor alleles were detected. During the blood meal, multiple vlsE alleles were observed in the tick. Tick feeding may increase recombination at the vlsE locus or selectively amplify rare vlsE alleles present in unfed ticks. On the basis of our data we propose a model, which is different from the established model for B. burgdorferi transmission. Implicit in our model is the concept that tick transmission converts a homogeneous spirochete population into a heterogeneous population that is poised to infect the mammalian host.
Figures


References
-
- Piesman J. Exp Appl Acarol. 1989;7:71–80. - PubMed
-
- Burgdorfer W, Lane R S, Barbour A G, Gresbrink R A, Anderson J R. Am J Trop Med Hyg. 1985;34:925–930. - PubMed
-
- Benach J L, Coleman J L, Skinner R A, Bosler E M. J Infect Dis. 1987;155:1300–1306. - PubMed
-
- Piesman J. J Infect Dis. 1993;167:1082–1085. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

