Estradiol rapidly inhibits soluble guanylyl cyclase expression in rat uterus
- PMID: 11209068
- PMCID: PMC14654
- DOI: 10.1073/pnas.98.2.717
Estradiol rapidly inhibits soluble guanylyl cyclase expression in rat uterus
Abstract
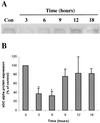
Previous reports that investigated the regulation of the NO/soluble guanylyl cyclase (sGC)/cGMP pathway by estrogenic compounds have focused primarily on the levels of NO, NO-producing enzymes, and cGMP in various tissues. In this study, we demonstrate that 17beta-estradiol (E2) regulates the alpha(1) and beta(1) subunits of the NO receptor, sGC, at the mRNA and protein levels in rat uterus. Using real-time quantitative PCR, we found that within 1 h of in vivo E2 administration to rats, sGC mRNA levels begin to diminish. After 3 h, there is a maximal diminution of sGC mRNA expression (sGC alpha(1) 10% and sGC beta(1) 33% of untreated). This effect was blocked by the estrogen receptor antagonist, ICI 182,780, indicating that estrogen receptor is required. The effect of E2 also was observed in vitro with incubations of uterine tissue, indicating that the response does not depend on the secondary release of other hormones or factors from other tissues. Puromycin did not block the effect, suggesting the effects occur because of preexisting factors in uterine tissues and do not require new protein synthesis. Using immunoblot analysis, we found that sGC protein levels also were reduced by E2 over a similar time course as the sGC mRNA. We conclude that sGC plays a vital role in the NO/sGC/cGMP regulatory pathway during conditions of elevated estrogen levels in the rat uterus as a result of the reduction of sGC expression.
Figures


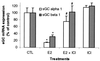
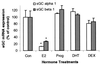
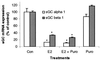

References
-
- Yallampalli C, Byam-Smith M, Nelson S O, Garfield R E. Endocrinology. 1994;134:1971–1974. - PubMed
-
- Brenner R M, Slayden O D. In: The Physiology of Reproduction. Knobil E, Neill J D, editors. New York: Raven; 1994. pp. 541–569.
-
- Burger H G. Horm Res. 2000;53:25–29. - PubMed
-
- Hyder S M, Kirkland J L, Loose-Mitchell D S, Makela S, Stancel G M. In: Endocrine Disruptors. Naz R K, editor. Boca Raton, FL: CRC; 1999. pp. 166–186.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

