Essential thioredoxin-dependent peroxiredoxin system from Helicobacter pylori: genetic and kinetic characterization
- PMID: 11222594
- PMCID: PMC95091
- DOI: 10.1128/JB.183.6.1961-1973.2001
Essential thioredoxin-dependent peroxiredoxin system from Helicobacter pylori: genetic and kinetic characterization
Abstract
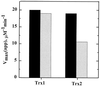
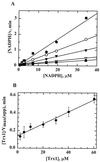
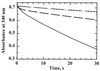
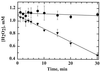
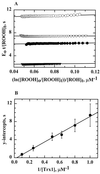
Helicobacter pylori, an oxygen-sensitive microaerophile, contains an alkyl hydroperoxide reductase homologue (AhpC, HP1563) that is more closely related to 2-Cys peroxiredoxins of higher organisms than to most other eubacterial AhpC proteins. Allelic replacement mutagenesis revealed ahpC to be essential, suggesting a critical role for AhpC in defending H. pylori against oxygen toxicity. Characterization of the ahpC promoter region divulged two putative regulatory elements and identified the transcription initiation site, which was mapped to 96 and 94 bp upstream of the initiation codon. No homologue of ahpF, which encodes the dedicated AhpC reductase in most eubacteria, was found in the H. pylori genome. Instead, homologues of Escherichia coli thioredoxin (Trx) reductase (TrxR, HP0825) and Trx (Trx1, HP0824) formed a reductase system for H. pylori AhpC. A second Trx homologue (Trx2, HP1458) was identified but was incapable of AhpC reduction, although Trx2 exhibited disulfide reductase activity with other substrates [insulin and 5,5'-dithiobis(2-nitrobenzoic acid)]. AhpC interactions with each substrate, Trx1 and hydroperoxide, were bimolecular and nonsaturable (infinite V(max) and K(m) values) but rapid enough (at 1 x 10(5) to 2 x 10(5) M(-1) s(-1)) to suggest an important role for AhpC in cellular peroxide metabolism. AhpC also exhibited a wide specificity for hydroperoxide substrates, which, taken together with the above results, suggests a minimal binding site for hydroperoxides composed of little more than the cysteinyl (Cys49) active site. H. pylori AhpC was not reduced by Salmonella typhimurium AhpF and was slightly more active with E. coli TrxR and Trx1 than was S. typhimurium AhpC, demonstrating the specialized catalytic properties of this peroxiredoxin.
Figures



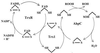
References
-
- Alphey M S, Bond C S, Tetaud E, Fairlamb A H, Hunter W N. The structure of reduced tryparedoxin peroxidase reveals a decamer and insight into reactivity of 2-Cys peroxiredoxins. J Mol Biol. 2000;300:903–916. - PubMed
-
- Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Ausubel F M, Brent R B, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Short protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1999.
-
- Baker L M S, Poole L B. An alkyl hydroperoxide reductase system from the gastric pathogen, Helicobacter pylori: cloning, purification, and kinetic studies. FASEB J. 1999;13:A1447.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

