D-alanylation of lipoteichoic acid: role of the D-alanyl carrier protein in acylation
- PMID: 11222605
- PMCID: PMC95102
- DOI: 10.1128/JB.183.6.2051-2058.2001
D-alanylation of lipoteichoic acid: role of the D-alanyl carrier protein in acylation
Abstract
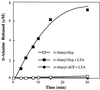
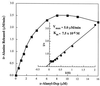
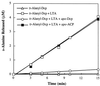
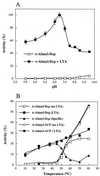
The D-alanylation of membrane-associated lipoteichoic acid (LTA) in gram-positive organisms requires the D-alanine-D-alanyl carrier protein ligase (AMP) (Dcl) and the D-alanyl carrier protein (Dcp). The dlt operon encoding these proteins (dltA and dltC) also includes dltB and dltD. dltB encodes a putative transport system, while dltD encodes a protein which facilitates the binding of Dcp and Dcl for ligation with D-alanine and has thioesterase activity for mischarged D-alanyl-acyl carrier proteins (ACPs). In previous results it was shown that D-alanyl-Dcp donates its ester residue to membrane-associated LTA (M. P. Heaton and F. C. Neuhaus, J. Bacteriol. 176: 681-690, 1994). However, all efforts to identify an enzyme which catalyzes this D-alanylation process were unsuccessful. It was discovered that incubation of D-alanyl-Dcp in the presence of LTA resulted in the time-dependent hydrolysis of this D-alanyl thioester. D-Alanyl-ACP in the presence of LTA was not hydrolyzed. When Dcp was incubated with membrane-associated D-alanyl LTA, a time and concentration-dependent formation of D-alanyl-Dcp was found. The addition of NaCl to this reaction inhibited the formation of D-alanyl-Dcp and stimulated the hydrolysis of D-alanyl-Dcp. Since these reactions are specific for the carrier protein (Dcp), it is suggested that Dcp has a unique binding site which interacts with the poly(Gro-P) moiety of LTA. It is this specific interaction that provides the functional specificity for the D-alanylation process. The reversibility of this process provides a mechanism for the transacylation of the D-alanyl ester residues between LTA and wall teichoic acid.
Figures




Similar articles
-
The D-Alanyl carrier protein in Lactobacillus casei: cloning, sequencing, and expression of dltC.J Bacteriol. 1996 Jul;178(13):3869-76. doi: 10.1128/jb.178.13.3869-3876.1996. J Bacteriol. 1996. PMID: 8682792 Free PMC article.
-
Biosynthesis of lipoteichoic acid in Lactobacillus rhamnosus: role of DltD in D-alanylation.J Bacteriol. 2000 May;182(10):2855-64. doi: 10.1128/JB.182.10.2855-2864.2000. J Bacteriol. 2000. PMID: 10781555 Free PMC article.
-
Incorporation of D-alanine into lipoteichoic acid and wall teichoic acid in Bacillus subtilis. Identification of genes and regulation.J Biol Chem. 1995 Jun 30;270(26):15598-606. doi: 10.1074/jbc.270.26.15598. J Biol Chem. 1995. PMID: 7797557
-
The dlt operon in the biosynthesis of D-alanyl-lipoteichoic acid in Lactobacillus casei.Microb Drug Resist. 1996 Spring;2(1):77-84. doi: 10.1089/mdr.1996.2.77. Microb Drug Resist. 1996. PMID: 9158726 Review.
-
A continuum of anionic charge: structures and functions of D-alanyl-teichoic acids in gram-positive bacteria.Microbiol Mol Biol Rev. 2003 Dec;67(4):686-723. doi: 10.1128/MMBR.67.4.686-723.2003. Microbiol Mol Biol Rev. 2003. PMID: 14665680 Free PMC article. Review.
Cited by
-
Mutant analysis and cellular localization of the AlgI, AlgJ, and AlgF proteins required for O acetylation of alginate in Pseudomonas aeruginosa.J Bacteriol. 2002 Jun;184(11):3000-7. doi: 10.1128/JB.184.11.3000-3007.2002. J Bacteriol. 2002. PMID: 12003941 Free PMC article.
-
Genome-scale model for Clostridium acetobutylicum: Part I. Metabolic network resolution and analysis.Biotechnol Bioeng. 2008 Dec 1;101(5):1036-52. doi: 10.1002/bit.22010. Biotechnol Bioeng. 2008. PMID: 18767192 Free PMC article.
-
Wall teichoic acids of gram-positive bacteria.Annu Rev Microbiol. 2013;67:313-36. doi: 10.1146/annurev-micro-092412-155620. Annu Rev Microbiol. 2013. PMID: 24024634 Free PMC article. Review.
-
Evidence that the algI/algJ gene cassette, required for O acetylation of Pseudomonas aeruginosa alginate, evolved by lateral gene transfer.J Bacteriol. 2004 Jul;186(14):4759-73. doi: 10.1128/JB.186.14.4759-4773.2004. J Bacteriol. 2004. PMID: 15231808 Free PMC article.
-
Genetic Loci of Plant Pathogenic Dickeya solani IPO 2222 Expressed in Contact with Weed-Host Bittersweet Nightshade (Solanum dulcamara L.) Plants.Int J Mol Sci. 2024 Feb 28;25(5):2794. doi: 10.3390/ijms25052794. Int J Mol Sci. 2024. PMID: 38474041 Free PMC article.
References
-
- Clemens D L, Kolenbrander P E, Debabov D V, Zhang Q, Lunsford R D, Sakone H, Whittaker C J, Heaton M P, Neuhaus F C. Insertional inactivation of genes responsible for the d-alanylation of lipoteichoic acid in Streptococcus gordonii DL1 (Challis) affects intrageneric coaggregations. Infect Immun. 1999;67:2464–2474. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

