Alpha-1-antichymotrypsin promotes beta-sheet amyloid plaque deposition in a transgenic mouse model of Alzheimer's disease
- PMID: 11222634
- PMCID: PMC6762932
- DOI: 10.1523/JNEUROSCI.21-05-01444.2001
Alpha-1-antichymotrypsin promotes beta-sheet amyloid plaque deposition in a transgenic mouse model of Alzheimer's disease
Abstract
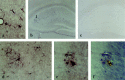
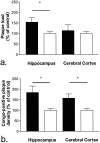
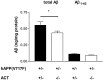
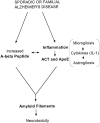
Alpha(1)-antichymotrypsin (ACT), an acute-phase inflammatory protein, is an integral component of the amyloid deposits in Alzheimer's disease (AD) and has been shown to catalyze amyloid beta-peptide polymerization in vitro. We have investigated the impact of ACT on amyloid deposition in vivo by generating transgenic GFAP-ACT-expressing mice and crossing them with the PDGF-hAPP/V717F mice, which deposit amyloid in an age-dependent manner. The number of amyloid deposits measured by Congo Red birefringence was increased in the double ACT/amyloid precursor protein (APP) transgenic mice compared with transgenic mice that only expressed APP, particularly in the hippocampus where ACT expression was highest, and the increase was preceded by elevated total amyloid beta-peptide levels at an early age. Our data demonstrate that ACT promotes amyloid deposition and provide a specific mechanism by which inflammation and the subsequent upregulation of astrocytic ACT expression in AD brain contributes to AD pathogenesis.
Figures


References
-
- Abraham CR, Selkoe DJ, Pottter H. Immunohistochemical identification of the serine protease inhibitor α1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell. 1988;52:487–501. - PubMed
-
- Abraham CR, Shirahama T, Potter H. α1-antichymotrypsin is associated solely with amyloid deposits containing the β-protein. Amyloid and cell localization of α1-antichymotrypsin. Neurobiol Aging. 1990;11:123–129. - PubMed
-
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. - PMC - PubMed
-
- Bales KR, Verina T, Dodel RC, Du Y, Altstiel L, Bender M, Hyslop P, Johnstone EM, Little SP, Cummins DJ, Piccardo P, Ghetti B, Paul SM. Lack of apolipoprotein E dramatically reduces amyloid β-peptide deposition. Nat Genet. 1997;17:263–264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
