Altered electrical properties in Drosophila neurons developing without synaptic transmission
- PMID: 11222642
- PMCID: PMC6762927
- DOI: 10.1523/JNEUROSCI.21-05-01523.2001
Altered electrical properties in Drosophila neurons developing without synaptic transmission
Abstract
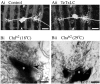
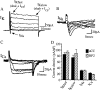
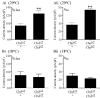
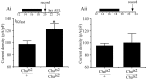
We examine the role of synaptic activity in the development of identified Drosophila embryonic motorneurons. Synaptic activity was blocked by both pan-neuronal expression of tetanus toxin light chain (TeTxLC) and by reduction of acetylcholine (ACh) using a temperature-sensitive allele of choline acetyltransferase (Cha(ts2)). In the absence of synaptic activity, aCC and RP2 motorneurons develop with an apparently normal morphology and retain their capacity to form synapses. However, blockade of synaptic transmission results in significant changes in the electrical phenotype of these neurons. Specifically, increases are seen in both voltage-gated inward Na(+) and voltage-gated outward K(+) currents. Voltage-gated Ca(2+) currents do not change. The changes in conductances appear to promote neuron excitability. In the absence of synaptic activity, the number of action potentials fired by a depolarizing ramp (-60 to +60 mV) is increased and, in addition, the amplitude of the initial action potential fired is also significantly larger. Silencing synaptic input to just aCC, without affecting inputs to other neurons, demonstrates that the capability to respond to changing levels of synaptic excitation is intrinsic to these neurons. The alteration to electrical properties are not permanent, being reversed by restoration of normal synaptic function. Whereas our data suggest that synaptic activity makes little or no contribution to the initial formation of embryonic neural circuits, the electrical development of neurons that constitute these circuits seems to depend on a process that requires synaptic activity.
Figures



References
-
- Bate M. Development of motor behaviour. Curr Opin Neurobiol. 1999;9:670–675. - PubMed
-
- Bito H. The role of calcium in activity-dependent neuronal gene expression. Cell Calcium. 1998;23:143–150. - PubMed
-
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
