Correlated firing in macaque visual area MT: time scales and relationship to behavior
- PMID: 11222658
- PMCID: PMC6762960
- DOI: 10.1523/JNEUROSCI.21-05-01676.2001
Correlated firing in macaque visual area MT: time scales and relationship to behavior
Abstract
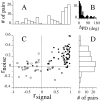
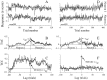
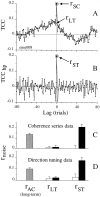
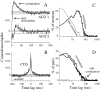
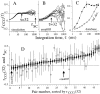
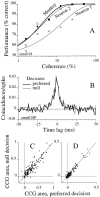
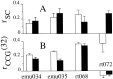
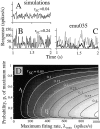
We studied the simultaneous activity of pairs of neurons recorded with a single electrode in visual cortical area MT while monkeys performed a direction discrimination task. Previously, we reported the strength of interneuronal correlation of spike count on the time scale of the behavioral epoch (2 sec) and noted its potential impact on signal pooling (Zohary et al., 1994). We have now examined correlation at longer and shorter time scales and found that pair-wise cross-correlation was predominantly short term (10-100 msec). Narrow, central peaks in the spike train cross-correlograms were largely responsible for correlated spike counts on the time scale of the behavioral epoch. Longer-term (many seconds to minutes) changes in the responsiveness of single neurons were observed in auto-correlations; however, these slow changes in time were on average uncorrelated between neurons. Knowledge of the limited time scale of correlation allowed the derivation of a more efficient metric for spike count correlation based on spike timing information, and it also revealed a potential relative advantage of larger neuronal pools for shorter integration times. Finally, correlation did not depend on the presence of the visual stimulus or the behavioral choice of the animal. It varied little with stimulus condition but was stronger between neurons with similar direction tuning curves. Taken together, our results strengthen the view that common input, common stimulus selectivity, and common noise are tightly linked in functioning cortical circuits.
Figures





References
-
- Abbott LF, Dayan P. The effect of correlated variability on the accuracy of a population code. Neural Comput. 1999;11:91–101. - PubMed
-
- Abeles M. Local cortical circuits: an electrophysiological study. Springer-Verlag; Berlin: 1982.
-
- Aertsen AMHJ, Gerstein GL, Habib MK, Palm G. Dynamics of neuronal firing correlation: modulation of “effective connectivity.”. J Neurophysiol. 1989;61:900–917. - PubMed
-
- Albright TD, Desimone R, Gross CG. Columnar organization of directionally selective cells in visual area MT of the macaque. J Neurophysiol. 1984;51:16–31. - PubMed
-
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
