Extrinsic modulation and motor pattern generation in a feeding network: a cellular study
- PMID: 11222666
- PMCID: PMC6762967
- DOI: 10.1523/JNEUROSCI.21-05-01767.2001
Extrinsic modulation and motor pattern generation in a feeding network: a cellular study
Abstract
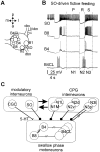
Systems level studies have shown that the paired serotonergic cerebral giant cells (CGCs) of gastropod mollusks have important extrinsic modulatory actions on the central pattern generator (CPG) underlying rhythmic ingestion movements. Here we present the first study that investigates the modulatory actions of the CGCs and their released transmitter 5-HT on the CPG at the cellular level. In the snail, Lymnaea, motoneurons such as the B4, B8, and B4CL cells are part of the feeding CPG and receive serotonergic synaptic inputs from CGCs. These motoneurons were used to investigate the effect of serotonergic modulation on endogenous cellular properties of CPG neurons. Cells were isolated from the intact nervous system, and their properties were examined by pharmacological methods in cell culture. Motoneurons were also grown in coculture with CGCs to compare 5-HT effects with CGC stimulation. Three distinct modulatory effects of exogenously applied 5-HT/CGC activity were seen: all three motoneuron types were depolarized by 5-HT for prolonged periods leading to firing. Conditional bursting accompanied this depolarization in the B4/B8 cells, but not in B4CL cells. The frequency of the bursting was increased with increased CGC tonic firing. An increase in the size of postinhibitory rebound (PIR) occurred with 5-HT application in all three cell types, because of an increase in a CsCl-sensitive, hyperpolarization-activated inward current. Similar modulatory effects on membrane potential, endogenous bursting, and PIR properties could be observed in the intact nervous system and were necessary for motoneuron activation during feeding. Part of the systems gating and frequency control functions of the CGCs appear to be caused by these modulatory effects on feeding motoneurons.
Figures






References
-
- Angstadt JD, Friesen WO. Modulation of swimming behaviour in the medicinal leech. I. Effects of serotonin on the electrical properties of swim-gating cell 204. J Comp Physiol [A] 1993a;172:223–234. - PubMed
-
- Angstadt JD, Friesen WO. Modulation of swimming behaviour in the medicinal leech. II. Ionic conductances underlying serotonergic modulation of swim-gating cell 204. J Comp Physiol [A] 1993b;172:235–248. - PubMed
-
- Benjamin PR, Elliott CJH. Snail feeding oscillator: the central pattern generator and its control by modulatory interneurons. In: Jacklet JW, editor. Neuronal and cellular oscillators. Marcel Dekker; New York: 1989. pp. 173–214.
-
- Benjamin PR, Rose RM. Central generation of bursting in the feeding system of the snail, Lymnaea stagnalis. J Exp Biol. 1979;80:93–118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
