Type D retrovirus Gag polyprotein interacts with the cytosolic chaperonin TRiC
- PMID: 11222675
- PMCID: PMC115874
- DOI: 10.1128/JVI.75.6.2526-2534.2001
Type D retrovirus Gag polyprotein interacts with the cytosolic chaperonin TRiC
Abstract
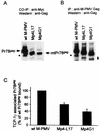
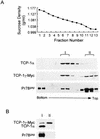
The carboxy terminus-encoding portion of the gag gene of Mason-Pfizer monkey virus (M-PMV), the prototype immunosuppressive primate type D retrovirus, encodes a 36-amino-acid, proline-rich protein domain that, in the mature virion, becomes the p4 capsid protein. The p4 domain has no known role in M-PMV replication. We found that two mutants with premature termination codons that remove half or all of the p4 domain produced lower levels of stable Gag protein and of self-assembled capsids. Interestingly, yeast two-hybrid screening revealed that p4 specifically interacted with TCP-1gamma, a subunit of the chaperonin TRiC (TCP-1 ring complex). TRiC is a cytosolic chaperonin that is known to be involved in both folding and subunit assembly of a variety of cellular proteins. TCP-1gamma also associated with high specificity with the M-PMV pp24/16-p12 domain and human immunodeficiency virus p6. Moreover, in cells, Gag polyprotein associated with the TRiC chaperonin complex and this association depended on ATP hydrolysis. In the p4 truncation mutants, the Gag-TRiC association was significantly reduced. These results strongly suggest that cytosolic chaperonin TRiC is involved in Gag folding and/or capsid assembly. We propose that TRiC associates transiently with nascent M-PMV Gag molecules to assist in their folding. Consequently, properly folded Gag molecules carry out the intermolecular interactions involved in self-assembly of the immature capsid.
Figures



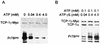
References
-
- Bachand F, Yao X J, Hrimech M, Rougeau N, Cohen É. Incorporation of Vpr into human immunodeficiency virus type 1 requires a direct interaction with the p6 domain of the p55 Gag precursor. J Biol Chem. 1999;274:9083–9091. - PubMed
-
- Bradac J, Hunter E. Polypeptides of Mason-Pfizer monkey virus. I. Synthesis and processing of the gag-gene products. Virology. 1984;138:260–275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

