Molecular biology of Barrett's adenocarcinoma
- PMID: 11224619
- PMCID: PMC1421247
- DOI: 10.1097/00000658-200103000-00005
Molecular biology of Barrett's adenocarcinoma
Abstract
Objective: To review the current knowledge on the genetic alterations involved in the development and progression of Barrett's esophagus-associated neoplastic lesions.
Summary background data: Barrett's esophagus (BE) is a premalignant condition in which the normal squamous epithelium of the esophagus is replaced by metaplastic columnar epithelium. BE predisposes patients to the development of esophageal adenocarcinoma. Endoscopic surveillance can detect esophageal adenocarcinomas when they are early and curable, but most of the adenocarcinomas are detected at an advanced stage. Despite advances in multimodal therapy, the prognosis for invasive esophageal adenocarcinoma is poor. A better understanding of the molecular evolution of the Barrett's metaplasia to dysplasia to adenocarcinoma sequence may allow improved diagnosis, therapy, and prognosis.
Methods: The authors reviewed data from the published literature to address what is known about the molecular changes thought to be important in the pathogenesis of BE-associated neoplastic lesions.
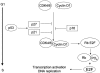
Results: The progression of Barrett's metaplasia to adenocarcinoma is associated with several changes in gene structure, gene expression, and protein structure. Some of the molecular alterations already showed promise as markers for early cancer detection or prognostication. Among these, alterations in the p53 and p16 genes and cell cycle abnormalities or aneuploidy appear to be the most important and well-characterized molecular changes. However, the exact sequence of events is not known, and probably multiple molecular pathways interact and are involved in the progression of BE to adenocarcinoma.
Conclusions: Further research into the molecular biology of BE-associated adenocarcinoma will enhance our understanding of the genetic events critical for the initiation and progression of Barrett's adenocarcinoma, leading to more effective surveillance and treatment.
Figures




References
-
- Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998; 83: 2049–2053. - PubMed
-
- Armstrong RW, Borman B. Trends in incidence rates of adenocarcinoma of the oesophagus and gastric cardia in New Zealand, 1978–1992. Int J Epidemiol 1996; 25: 941–947. - PubMed
-
- Hansson LE, Sparen P, Nyren O. Increasing incidence of both major histological types of esophageal carcinomas among men in Sweden. Int J Cancer 1993; 54: 402–407. - PubMed
-
- Hansen S, Wiig JN, Giercksky KE, et al. Esophageal and gastric carcinoma in Norway 1958–1992: incidence time trend variability according to morphological subtypes and organ subsites. Int J Cancer 1997; 71: 340–344. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

