Glucuronide and glucoside conjugation of mycophenolic acid by human liver, kidney and intestinal microsomes
- PMID: 11226133
- PMCID: PMC1572641
- DOI: 10.1038/sj.bjp.0703898
Glucuronide and glucoside conjugation of mycophenolic acid by human liver, kidney and intestinal microsomes
Abstract
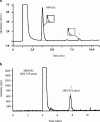
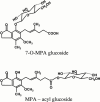
Mycophenolic acid (MPA) is primarily metabolized to a phenolic glucuronide (MPAG) as well as to two further minor metabolites: an acyl glucuronide (AcMPAG) and a phenolic glucoside (MPAG1s). This study presents investigations of the formation of these metabolites by human liver (HLM), kidney (HKM), and intestinal (HIM) microsomes, as well as by recombinant UDP-glucuronosyltransferases. HLM (n=5), HKM (n=6), HIM (n=5) and recombinant UGTs were incubated in the presence of either UDP-glucuronic acid or UDP-glucose and various concentrations of MPA. Metabolite formation was followed by h.p.l.c. All microsomes investigated formed both MPAG and AcMPAG. Whereas the efficiency of MPAG formation was greater with HKM compared to HLM, AcMPAG formation was greater with HLM than HKM. HIM showed the lowest glucuronidation efficiency and the greatest interindividual variation. The capacity for MPAGls formation was highest in HKM, while no glucoside was detected with HIM. HKM produced a second metabolite when incubated with MPA and UDP-glucose, which was labile to alkaline treatment. Mass spectrometry of this metabolite in the negative ion mode revealed a molecular ion of m/z 481 compatible with an acyl glucoside conjugate of MPA. All recombinant UGTs investigated were able to glucuronidate MPA with K:(M:) values ranging from 115.3 to 275.7 microM l(-1) and V(max) values between 29 and 106 pM min(-1) mg protein(-1). Even though the liver is the most important site of MPA glucuronidation, extrahepatic tissues particularly the kidney may play a significant role in the overall biotransformation of MPA in man. Only kidney microsomes formed a putative acyl glucoside of MPA.
Figures
References
-
- BEHREND M. Mycophenolate mofetil (Cellcept®) Exp. Opin. Invest. Drugs. 1998;7:1509–1519. - PubMed
-
- BRIGGS W.A., CHOI M.J., SCHEEL P.J., JR Successful mycophenolate mofetil treatment of glomerular disease. Am. J. Kidney Dis. 1998;31:213–217. - PubMed
-
- BULLINGHAM R., MONROE S., NICHOLLS A., HALE M. Pharmacokinetics and bioavailability of mycophenolate mofetil in healthy subjects after single-dose oral and intravenous administration. Clin. Pharmacokinet. 1998;34:315–324. - PubMed
-
- BULLINGHAM R.E.S., NICHOLLS A.J., KAMM B.R. Clinical Pharmacokinetics of mycophenolate mofetil. Clin. Pharmacokinet. 1998;34:429–455. - PubMed
-
- ENK A.H., KNOP J. Treatment of pemphigus vulgaris with mycophenolate mofetil [letter] Lancet. 1997;350:494. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

