Pharmacological differences between the human and rat vanilloid receptor 1 (VR1)
- PMID: 11226139
- PMCID: PMC1572656
- DOI: 10.1038/sj.bjp.0703918
Pharmacological differences between the human and rat vanilloid receptor 1 (VR1)
Abstract
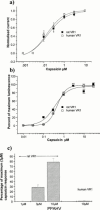
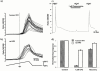
Vanilloid receptors (VR1) were cloned from human and rat dorsal root ganglion libraries and expressed in Xenopus oocytes or Chinese Hamster Ovary (CHO) cells. Both rat and human VR1 formed ligand gated channels that were activated by capsaicin with similar EC(50) values. Capsaicin had a lower potency on both channels, when measured electrophysiologically in oocytes compared to CHO cells (oocytes: rat=1.90+/-0.20 microM; human=1.90+/-0.30 microM: CHO cells: rat=0.20+/-0.06 microM; human=0.19+/-0.08 microM). In CHO cell lines co-expressing either rat or human VR1 and the calcium sensitive, luminescent protein, aequorin, the EC(50) values for capsaicin-induced responses were similar in both cell lines (rat=0.35+/-0.06 microM, human=0.53+/-0.03 microM). The threshold for activation by acidic solutions was lower for human VR1 channels than that for rat VR1 (EC(50) pH 5.49+/-0.04 and pH 5.78+/-0.09, respectively). The threshold for heat activation was identical (42 degrees C) for rat and human VR1. PPAHV was an agonist at rat VR1 (EC(50) between 3 and 10 microM) but was virtually inactive at the human VR1 (EC(50)>10 microM). Capsazepine and ruthenium red were both more potent at blocking the capsaicin response of human VR1 than rat VR1. Capsazepine blocked the human but not the rat VR1 response to low pH. Capsazepine was also more effective at inhibiting the noxious heat response of human than of rat VR1.
Figures

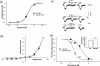


References
-
- APPENDINO G., CRAVOTTO G., PALMISANO G., ANNUNZIATA R., SZALLASI A. Synthesis and evaluation of phorboid 20-homovanillates: discovery of a class of ligands binding to the vanilloid (capsaicin) receptor with different degrees of cooperativity. J. Med. Chem. 1996;39:3123–3131. - PubMed
-
- BAUMANN T.K., BURCHIEL K.J., INGRAM S.L., MARTENSON M.E. Responses of adult dorsal root ganglion neurones in culture to capsaicin and low pH. Pain. 1996;65:31–38. - PubMed
-
- BEVAN S., DOCHERTY R.J.Cellular mechanisms of the action of capsaicin Capsaicin in the study of pain 1993London: Academic Press; 27–44.ed. Wood, J.N. pp
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

