Structure of a genetically engineered molecular motor
- PMID: 11226153
- PMCID: PMC140180
- DOI: 10.1093/emboj/20.1.40
Structure of a genetically engineered molecular motor
Abstract
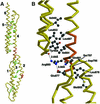
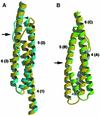
Molecular motors move unidirectionally along polymer tracks, producing movement and force in an ATP-dependent fashion. They achieve this by amplifying small conformational changes in the nucleotide-binding region into force-generating movements of larger protein domains. We present the 2.8 A resolution crystal structure of an artificial actin-based motor. By combining the catalytic domain of myosin II with a 130 A conformational amplifier consisting of repeats 1 and 2 of alpha-actinin, we demonstrate that it is possible to genetically engineer single-polypeptide molecular motors with precisely defined lever arm lengths and specific motile properties. Furthermore, our structure shows the consequences of mutating a conserved salt bridge in the nucleotide-binding region. Disruption of this salt bridge, which is known to severely inhibit ATP hydrolysis activity, appears to interfere with formation of myosin's catalytically active 'closed' conformation. Finally, we describe the structure of alpha-actinin repeats 1 and 2 as being composed of two rigid, triple-helical bundles linked by an uninterrupted alpha-helix. This fold is very similar to the previously described structures of alpha-actinin repeats 2 and 3, and alpha-spectrin repeats 16 and 17.
Figures

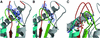

References
-
- Blanchard A., Ohanian,V. and Critchley,D. (1989) The structure and function of α-actinin. J. Muscle Res. Cell Motil., 10, 280–289. - PubMed
-
- Brunger A.T. et al. (1998) Crystallography and NMR system (CNS): a new software system for macromolecular structure determination. Acta Crystallogr. D, 54, 905–921. - PubMed
-
- Burley S.K. et al (1999) Structural genomics: beyond the human genome project. Nature Genet., 23, 151–157. - PubMed
-
- Corrie J.E. et al. (1999) Dynamic measurement of myosin light-chain-domain tilt and twist in muscle contraction. Nature, 400, 425–430. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

