Role of elementary Ca(2+) puffs in generating repetitive Ca(2+) oscillations
- PMID: 11226156
- PMCID: PMC140189
- DOI: 10.1093/emboj/20.1.65
Role of elementary Ca(2+) puffs in generating repetitive Ca(2+) oscillations
Abstract
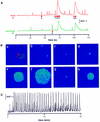
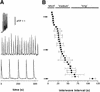
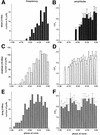
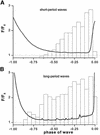
Inositol (1,4,5)-trisphosphate (IP(3)) liberates intracellular Ca(2+) both as localized 'puffs' and as repetitive waves that encode information in a frequency-dependent manner. Using video-rate confocal imaging, together with photorelease of IP(3) in Xenopus oocytes, we investigated the roles of puffs in determining the periodicity of global Ca(2+) waves. Wave frequency is not delimited solely by cyclical recovery of the cell's ability to support wave propagation, but further involves sensitization of Ca(2+)-induced Ca(2+) release by progressive increases in puff frequency and amplitude at numerous sites during the interwave period, and accumulation of pacemaker Ca(2+), allowing a puff at a 'focal' site to trigger a subsequent wave. These specific 'focal' sites, distinguished by their higher sensitivity to IP(3) and close apposition to neighboring puff sites, preferentially entrain both the temporal frequency and spatial directionality of Ca(2+) waves. Although summation of activity from many stochastic puff sites promotes the generation of regularly periodic global Ca(2+) signals, the properties of individual Ca(2+) puffs control the kinetics of Ca(2+) spiking and the (higher) frequency of subcellular spikes in their local microdomain.
Figures




References
-
- Berridge M.J. (1997a) The AM and FM of calcium signalling. Nature, 386, 759–760. - PubMed
-
- Berridge M.J. and Dupont,G. (1994) Spatial and temporal signalling by calcium. Curr. Opin. Cell Biol., 6, 267–274. - PubMed
-
- Bezprozvanny I., Watras,J. and Ehrlich,B.E. (1991) Bell-shaped calcium-response curves for Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature, 351, 751–754. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

