Wall-associated kinases are expressed throughout plant development and are required for cell expansion
- PMID: 11226187
- PMCID: PMC102244
- DOI: 10.1105/tpc.13.2.303
Wall-associated kinases are expressed throughout plant development and are required for cell expansion
Abstract
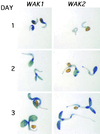
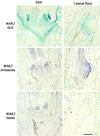
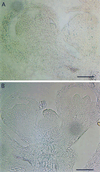
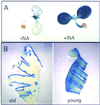
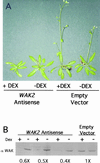
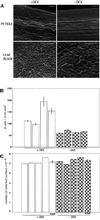
The mechanism by which events in the angiosperm cell wall are communicated to the cytoplasm is not well characterized. A family of five Arabidopsis wall-associated kinases (WAKs) have the potential to provide a physical and signaling continuum between the cell wall and the cytoplasm. The WAKs have an active cytoplasmic protein kinase domain, span the plasma membrane, and contain an N terminus that binds the cell wall. We show here that WAKs are expressed at organ junctions, in shoot and root apical meristems, in expanding leaves, and in response to wall disturbances. Leaves expressing an antisense WAK gene have reduced WAK protein levels and exhibit a loss of cell expansion. WAKs are covalently bound to pectin in the cell wall, providing evidence that the binding of a structural carbohydrate by a receptor-like kinase may have significance in the control of cell expansion.
Figures



References
-
- Alvarez, M.E., Pennell, R.I., Meijer, P.-J., Ishikawa, A., Dixon, R.A., and Lamb, C. (1998). Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92, 773–784. - PubMed
-
- Bleecker, A.B., Estelle, M.A., Somerville, C., and Kende, H. (1988). Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 241, 1086–1089. - PubMed
-
- Butt, A., Mousley, C., Morris, K., Beynon, J., Can, C., Holub, E., Greenberg, J.T., and Buchanan-Wollastonq, V. (1998). Differential expression of a senescence-enhanced metallothionein gene in Arabidopsis in response to isolates of Peronospora parasitica and Pseudomonas syringae. Plant J. 16, 209–221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

