The origin of atmospheric oxygen on Earth: the innovation of oxygenic photosynthesis
- PMID: 11226211
- PMCID: PMC30111
- DOI: 10.1073/pnas.061514798
The origin of atmospheric oxygen on Earth: the innovation of oxygenic photosynthesis
Abstract
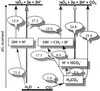
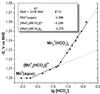
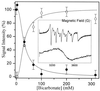
The evolution of O(2)-producing cyanobacteria that use water as terminal reductant transformed Earth's atmosphere to one suitable for the evolution of aerobic metabolism and complex life. The innovation of water oxidation freed photosynthesis to invade new environments and visibly changed the face of the Earth. We offer a new hypothesis for how this process evolved, which identifies two critical roles for carbon dioxide in the Archean period. First, we present a thermodynamic analysis showing that bicarbonate (formed by dissolution of CO(2)) is a more efficient alternative substrate than water for O(2) production by oxygenic phototrophs. This analysis clarifies the origin of the long debated "bicarbonate effect" on photosynthetic O(2) production. We propose that bicarbonate was the thermodynamically preferred reductant before water in the evolution of oxygenic photosynthesis. Second, we have examined the speciation of manganese(II) and bicarbonate in water, and find that they form Mn-bicarbonate clusters as the major species under conditions that model the chemistry of the Archean sea. These clusters have been found to be highly efficient precursors for the assembly of the tetramanganese-oxide core of the water-oxidizing enzyme during biogenesis. We show that these clusters can be oxidized at electrochemical potentials that are accessible to anoxygenic phototrophs and thus the most likely building blocks for assembly of the first O(2) evolving photoreaction center, most likely originating from green nonsulfur bacteria before the evolution of cyanobacteria.
Figures


References
-
- Des Marais D J. Science. 2000;289:1703–1704. - PubMed
-
- Holland H, Rye R. Am J Sci. 1998;298:621–672. - PubMed
-
- Debus R J. Biochim Biophys Acta. 1992;1102:269–352. - PubMed
-
- Debus R. In: Metal Ions in Biological Systems. Sigel H, editor. Vol. 37. New York: Dekker; 2000. pp. 657–710. - PubMed
-
- Mulkidjanian A Y, Junge W. Photosynth Res. 1997;51:27–42.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

