The human mitochondrial deoxynucleotide carrier and its role in the toxicity of nucleoside antivirals
- PMID: 11226231
- PMCID: PMC30130
- DOI: 10.1073/pnas.031430998
The human mitochondrial deoxynucleotide carrier and its role in the toxicity of nucleoside antivirals
Abstract
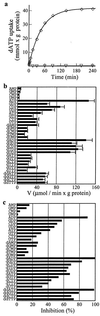
The synthesis of DNA in mitochondria requires the uptake of deoxynucleotides into the matrix of the organelle. We have characterized a human cDNA encoding a member of the family of mitochondrial carriers. The protein has been overexpressed in bacteria and reconstituted into phospholipid vesicles where it catalyzed the transport of all four deoxy (d) NDPs, and, less efficiently, the corresponding dNTPs, in exchange for dNDPs, ADP, or ATP. It did not transport dNMPs, NMPs, deoxynucleosides, nucleosides, purines, or pyrimidines. The physiological role of this deoxynucleotide carrier is probably to supply deoxynucleotides to the mitochondrial matrix for conversion to triphosphates and incorporation into mitochondrial DNA. The protein is expressed in all human tissues that were examined except for placenta, in accord with such a central role. The deoxynucleotide carrier also transports dideoxynucleotides efficiently. It is likely to be medically important by providing the means of uptake into mitochondria of nucleoside analogs, leading to the mitochondrial impairment that underlies the toxic side effects of such drugs in the treatment of viral illnesses, including AIDS, and in cancer therapy.
Figures



References
-
- Walker J E. Curr Opin Struct Biol. 1992;2:519–526.
-
- Palmieri F. FEBS Lett. 1994;346:48–54. - PubMed
-
- Palmieri F, van Ommen B. In: Frontiers in Cellular Bioenergetics. Papa S, Guerrieri F, Tager J M, editors. New York: Kluwer Academic/Plenum; 1999. pp. 489–519.
-
- Palmieri L, De Marco V, Iacobazzi V, Palmieri F, Runswick M J, Walker J E. FEBS Lett. 1997;410:447–451. - PubMed
-
- Palmieri L, Lasorsa F M, De Palma A, Palmieri F, Runswick M J, Walker J E. FEBS Lett. 1997;417:114–118. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

