Transverse relaxation-optimized NMR spectroscopy with the outer membrane protein OmpX in dihexanoyl phosphatidylcholine micelles
- PMID: 11226244
- PMCID: PMC30143
- DOI: 10.1073/pnas.051629298
Transverse relaxation-optimized NMR spectroscopy with the outer membrane protein OmpX in dihexanoyl phosphatidylcholine micelles
Abstract
The (2)H,(13)C,(15)N-labeled, 148-residue integral membrane protein OmpX from Escherichia coli was reconstituted with dihexanoyl phosphatidylcholine (DHPC) in mixed micelles of molecular mass of about 60 kDa. Transverse relaxation-optimized spectroscopy (TROSY)-type triple resonance NMR experiments and TROSY-type nuclear Overhauser enhancement spectra were recorded in 2 mM aqueous solutions of these mixed micelles at pH 6.8 and 30 degrees C. Complete sequence-specific NMR assignments for the polypeptide backbone thus have been obtained. The (13)C chemical shifts and the nuclear Overhauser effect data then resulted in the identification of the regular secondary structure elements of OmpX/DHPC in solution and in the collection of an input of conformational constraints for the computation of the global fold of the protein. The same type of polypeptide backbone fold is observed in the presently determined solution structure and the previously reported crystal structure of OmpX determined in the presence of the detergent n-octyltetraoxyethylene. Further structure refinement will have to rely on the additional resonance assignment of partially or fully protonated amino acid side chains, but the present data already demonstrate that relaxation-optimized NMR techniques open novel avenues for studies of structure and function of integral membrane proteins.
Figures
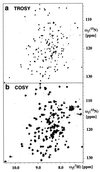



References
-
- Walker J E, Saraste M. Curr Opin Struct Biol. 1996;6:457–459. - PubMed
-
- Kühlbrandt W, Gouaux E. Curr Opin Struct Biol. 1999;9:445–447. - PubMed
-
- Bowie J. Curr Opin Struct Biol. 2000;10:435–437. - PubMed
-
- Wüthrich K. NMR of Proteins and Nucleic Acids. New York: Wiley; 1986.
-
- Cavanagh J, Fairbrother W J, Palmer A G, III, Skelton N J. Protein NMR Spectroscopy. Principles and Practice. New York: Academic; 1996.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

