Organellar relationships in the Golgi region of the pancreatic beta cell line, HIT-T15, visualized by high resolution electron tomography
- PMID: 11226251
- PMCID: PMC30150
- DOI: 10.1073/pnas.051631998
Organellar relationships in the Golgi region of the pancreatic beta cell line, HIT-T15, visualized by high resolution electron tomography
Abstract
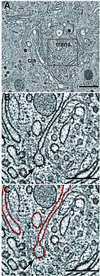
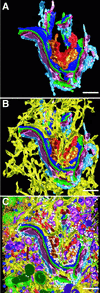
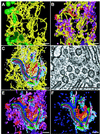
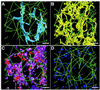
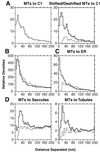
The positional relationships among all of the visible organelles in a densely packed region of cytoplasm from an insulin secreting, cultured mammalian cell have been analyzed in three dimensions (3-D) at approximately 6 nm resolution. Part of a fast frozen/freeze-substituted HIT-T15 cell that included a large portion of the Golgi ribbon was reconstructed in 3-D by electron tomography. The reconstructed volume (3.1 x 3.2 x 1.2 microm(3)) allowed sites of interaction between organelles, and between microtubules and organellar membranes, to be accurately defined in 3-D and quantitatively analyzed by spatial density analyses. Our data confirm that the Golgi in an interphase mammalian cell is a single, ribbon-like organelle composed of stacks of flattened cisternae punctuated by openings of various sizes [Rambourg, A., Clermont, Y., & Hermo, L. (1979) Am. J. Anat. 154, 455-476]. The data also show that the endoplasmic reticulum (ER) is a single continuous compartment that forms close contacts with mitochondria, multiple trans Golgi cisternae, and compartments of the endo-lysosomal system. This ER traverses the Golgi ribbon from one side to the other via cisternal openings. Microtubules form close, non-random associations with the cis Golgi, the ER, and endo-lysosomal compartments. Despite the dense packing of organelles in this Golgi region, approximately 66% of the reconstructed volume is calculated to represent cytoplasmic matrix. We relate the intimacy of structural associations between organelles in the Golgi region, as quantified by spatial density analyses, to biochemical mechanisms for membrane trafficking and organellar communication in mammalian cells.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

