IL-4 inhibits osteoclast formation through a direct action on osteoclast precursors via peroxisome proliferator-activated receptor gamma 1
- PMID: 11226258
- PMCID: PMC30157
- DOI: 10.1073/pnas.041493198
IL-4 inhibits osteoclast formation through a direct action on osteoclast precursors via peroxisome proliferator-activated receptor gamma 1
Abstract
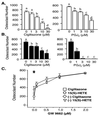
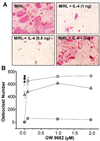
IL-4 is a pleiotropic immune cytokine secreted by activated T(H)2 cells that inhibits bone resorption both in vitro and in vivo. The cellular targets of IL-4 action as well as its intracellular mechanism of action remain to be determined. We show here that IL-4 inhibits receptor activator of NF-kappaB ligand-induced osteoclast differentiation through an action on osteoclast precursors that is independent of stromal cells. Interestingly, this inhibitory effect can be mimicked by both natural as well as synthetic peroxisome proliferator-activated receptor gamma1 (PPARgamma1) ligands and can be blocked by the irreversible PPARgamma antagonist GW 9662. These findings suggest that the actions of IL-4 on osteoclast differentiation are mediated by PPARgamma1, an interpretation strengthened by the observation that IL-4 can activate a PPARgamma1-sensitive luciferase reporter gene in RAW264.7 cells. We also show that inhibitors of enzymes such as 12/15-lipoxygenase and the cyclooxygenases that produce known PPARgamma1 ligands do not abrogate the IL-4 effect. These findings, together with the observation that bone marrow cells from 12/15-lipoxygenase-deficient mice retain sensitivity to IL-4, suggest that the cytokine may induce novel PPARgamma1 ligands. Our results reveal that PPARgamma1 plays an important role in the suppression of osteoclast formation by IL-4 and may explain the beneficial effects of the thiazolidinedione class of PPARgamma1 ligands on bone loss in diabetic patients.
Figures


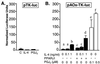
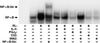

References
-
- Manolagas S C, Jilka R L. N Engl J Med. 1995;332:305–311. - PubMed
-
- Turner R T, Riggs B L, Spelsberg T C. Endocr Rev. 1994;15:275–300. - PubMed
-
- Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. San Diego: Academic; 1996.
-
- Roodman G D. Exp Hematol. 1999;27:1229–1241. - PubMed
-
- Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie M T, Martin T J. Endocr Rev. 1999;20:345–357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

