Interaction of a transcriptional repressor with the RNA polymerase II holoenzyme plays a crucial role in repression
- PMID: 11226276
- PMCID: PMC30175
- DOI: 10.1073/pnas.041611198
Interaction of a transcriptional repressor with the RNA polymerase II holoenzyme plays a crucial role in repression
Abstract
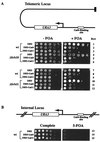
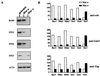
The yeast transcriptional repressor Tup1, tethered to DNA, represses to strikingly different degrees transcription elicited by members of two classes of activators. Repression in both cases is virtually eliminated by mutation of either member of the cyclin-kinase pair Srb10/11. In contrast, telomeric chromatin affects both classes of activators equally, and in neither case is that repression affected by mutation of Srb10/11. In vitro, Tup1 interacts with RNA polymerase II holoenzyme bearing Srb10 as well as with the separated Srb10. These and other findings indicate that at least one aspect of Tup1's action involves interaction with the RNA polymerase II holoenzyme.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

