Synergistic contributions of cyclin-dependant kinase 5/p35 and Reelin/Dab1 to the positioning of cortical neurons in the developing mouse brain
- PMID: 11226314
- PMCID: PMC30213
- DOI: 10.1073/pnas.051628498
Synergistic contributions of cyclin-dependant kinase 5/p35 and Reelin/Dab1 to the positioning of cortical neurons in the developing mouse brain
Abstract
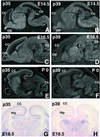
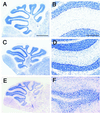
Cyclin-dependent kinase (Cdk) 5 is a unique member of the Cdk family, because Cdk5 kinase activity is detected only in the nervous tissue. Two neuron-specific activating subunits of Cdk5, p35 and p39, have been identified. Overlapping expression pattern of these isoforms in the embryonic mouse brain and the significant residual Cdk5 kinase activity in brain homogenate of the p35-/- mice indicate the redundant functions of the Cdk5 activators in vivo. Severe neuronal migration defects in p35-/-Cdk5 +/- mice further support the idea that the redundant expression of the Cdk5 activators may cause a milder phenotype in p35-/- mice compared with Cdk5-/- mice. Mutant mice lacking either Cdk5 or p35 exhibit certain similarities with Reelin/Dab1-mutant mice in the disorganization of cortical laminar structure in the brain. To elucidate the relationship between Cdk5/p35 and Reelin/Dab1 signaling, we generated mouse lines that have combined defects of these genes. The addition of heterozygosity of either Dab1 or Reelin mutation to p35-/- causes the extensive migration defects of cortical neurons in the cerebellum. In the double-null mice of p35 and either Dab1 or Reelin, additional migration defects occur in the Purkinje cells in the cerebellum and in the pyramidal neurons in the hippocampus. These additional defects in neuronal migration in mice lacking both Cdk5/p35 and Reelin/Dab1 indicate that Cdk5/p35 may contribute synergistically to the positioning of the cortical neurons in the developing mouse brain.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

