Characteristics of glycine receptors expressed by embryonic rat brain mRNAs
- PMID: 11226317
- PMCID: PMC30216
- DOI: 10.1073/pnas.031580798
Characteristics of glycine receptors expressed by embryonic rat brain mRNAs
Abstract
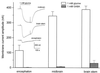
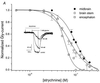
A study was made of glycine (Gly) and gamma-aminobutyric acid (GABA) receptors expressed in Xenopus oocytes injected with rat mRNAs isolated from the encephalon, midbrain, and brainstem of 18-day-old rat embryos. In oocytes injected with encephalon, midbrain, or brainstem mRNAs, the Gly-current amplitudes (membrane current elicited by Gly; 1 mM Gly) were respectively 115 +/- 35, 346 +/- 28, and 389 +/- 22 nA, whereas the GABA-currents (1 mM GABA) were all < or =40 nA. Moreover, the Gly-currents desensitized faster in oocytes injected with encephalon or brainstem mRNAs. The EC(50) for Gly was 611 +/- 77 microM for encephalon, 661 +/- 28 microM for midbrain, and 506 +/- 18 microM for brainstem mRNA-injected oocytes, and the corresponding Hill coefficients were all approximately 2. Strychnine inhibited all of the Gly-currents, with an IC(50) of 56 +/- 3 nM for encephalon, 97 +/- 4 nM for midbrain, and 72 +/- 4 nM for brainstem mRNAs. During repetitive Gly applications, the Gly-currents were potentiated by 1.6-fold for encephalon, 2.1-fold for midbrain, and 1.3-fold for brainstem RNA-injected oocytes. Raising the extracellular Ca(2+) concentration significantly increased the Gly-currents in oocytes injected with midbrain and brainstem mRNAs. Reverse transcription-PCR studies showed differences in the Gly receptor (GlyR) alpha-subunits expressed, whereas the beta-subunit was present in all three types of mRNA. These results indicate differential expression of GlyR mRNAs in the brain areas examined, and these mRNAs lead to the expression of GlyRs that have different properties. The modulation of GlyRs by Ca(2+) could play important functions during brain development.
Figures





Similar articles
-
Cloning and functional expression of the bovine GABA(C) rho2 subunit. Molecular evidence of a widespread distribution in the CNS.Neurosci Res. 2005 Dec;53(4):421-7. doi: 10.1016/j.neures.2005.08.014. Epub 2005 Oct 4. Neurosci Res. 2005. PMID: 16213047
-
mRNAs coding for neurotransmitter receptors in rabbit and rat visual areas.J Neurosci Res. 1993 Aug 15;35(6):652-63. doi: 10.1002/jnr.490350608. J Neurosci Res. 1993. PMID: 8411267
-
Ethanol potentiation of glycine receptors expressed in Xenopus oocytes antagonized by increased atmospheric pressure.Alcohol Clin Exp Res. 2003 May;27(5):743-55. doi: 10.1097/01.ALC.0000065722.31109.A1. Alcohol Clin Exp Res. 2003. PMID: 12766618
-
Inhibitory glycine receptors: an update.J Biol Chem. 2012 Nov 23;287(48):40216-23. doi: 10.1074/jbc.R112.408229. Epub 2012 Oct 4. J Biol Chem. 2012. PMID: 23038260 Free PMC article. Review.
-
[Comparative biochemistry of the triune brain].Acta Psychiatr Belg. 1994;94(4-6):225-47. Acta Psychiatr Belg. 1994. PMID: 8525844 Review. French.
References
-
- Betz H, Kuhse J, Schmieden V, Laube B, Kirsch J, Harvey R J. Ann NY Acad Sci. 1999;868:667–676. - PubMed
-
- Bormann I. Trends Pharmacol Sci. 2000;21:16–19. - PubMed
-
- Mehta A K, Ticku M K. Brain Res Rev. 1999;29:196–217. - PubMed
-
- Lukas R J, Changeux J-P, Le Novere N, Albuquerque E X, Balfour D J K, Kerg D K, Bertrand D, Chiappinelli V A, Clarke P B, Collins A C, et al. Pharmacol Rev. 1999;51:397–401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

