beta -Neuregulin-1 is required for the in vivo development of functional Ca2+-activated K+ channels in parasympathetic neurons
- PMID: 11226326
- PMCID: PMC30225
- DOI: 10.1073/pnas.041394098
beta -Neuregulin-1 is required for the in vivo development of functional Ca2+-activated K+ channels in parasympathetic neurons
Abstract
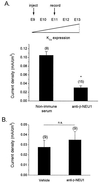
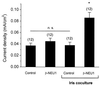
The development of functional Ca(2+)-activated K(+) channels (K(Ca)) in chick ciliary ganglion (CG) neurons requires interactions with afferent preganglionic nerve terminals. Here we show that the essential preganglionic differentiation factor is an isoform of beta-neuregulin-1. beta-Neuregulin-1 transcripts are expressed in the midbrain preganglionic Edinger-Westphal nucleus at developmental stages that coincide with or precede the normal onset of macroscopic K(Ca) in CG neurons. Injection of beta-neuregulin-1 peptide into the brains of developing embryos evoked a robust stimulation of functional K(Ca) channels at stages before the normal appearance of these channels in CG neurons developing in vivo. Conversely, injection of a neutralizing antiserum specific for beta-neuregulin-1 inhibited the development of K(Ca) channels in CG neurons. Low concentrations of beta-neuregulin-1 evoked a robust increase in whole-cell K(Ca) in CG neurons cocultured with iris target tissues. By contrast, culturing CG neurons with iris cells or low concentrations of beta-neuregulin-1 by themselves was insufficient to stimulate K(Ca). These data suggest that the preganglionic factor required for the development of K(Ca) in ciliary ganglion neurons is an isoform of beta-neuregulin-1, and that this factor acts in concert with target-derived trophic molecules to regulate the differentiation of excitability.
Figures



References
-
- Fischbach G D, Rosen K M. Annu Rev Neurosci. 1997;20:429–458. - PubMed
-
- Gassmann M, Lemke G. Curr Opin Neurobiol. 1997;7:87–92. - PubMed
-
- Falls D L, Rosen K M, Corfas G, Lane W S, Fischbach G D. Cell. 1993;72:801–815. - PubMed
-
- Sandrock A W, Dryer S E, Rosen K M, Gozani S N, Kramer R, Theill L E, Fischbach G D. Science. 1997;276:599–603. - PubMed
-
- Yang X, Kuo Y, Devay P, Yu C, Role L. Neuron. 1998;20:255–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

