The three modules of ADP/ATP carrier cooperate in receptor recruitment and translocation into mitochondria
- PMID: 11230119
- PMCID: PMC145466
- DOI: 10.1093/emboj/20.5.951
The three modules of ADP/ATP carrier cooperate in receptor recruitment and translocation into mitochondria
Abstract
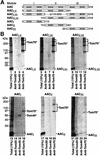
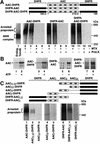
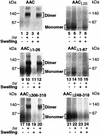
The ADP/ATP carrier (AAC) is a major representative of mitochondrial preproteins lacking an N-terminal presequence. AAC contains targeting information in each of its three modules, which has led to a search for the dominant targeting region. An alternative, not yet tested model would be that several distinct targeting signals function simultaneously in import of the preprotein. We report that the three AAC modules cooperate in binding to the receptor Tom70 such that three Tom70 dimers are recruited to one preprotein. The modules are transferred to the import pore in a stepwise manner and cooperate again in the accumulation of AAC in the general import pore complex. AAC can cross the outer membrane with an internal segment first, i.e. in a loop formation. Each module of AAC is required for dimerization in the inner membrane. We propose a new concept for import of the hydrophobic carrier proteins into mitochondria where multiple signals cooperate in receptor recruitment, outer membrane translocation via loop formation and assembly in the inner membrane.
Figures


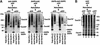
Similar articles
-
Transport of the ADP/ATP carrier of mitochondria from the TOM complex to the TIM22.54 complex.EMBO J. 1999 Jun 15;18(12):3214-21. doi: 10.1093/emboj/18.12.3214. EMBO J. 1999. PMID: 10369662 Free PMC article.
-
Tom22 is a multifunctional organizer of the mitochondrial preprotein translocase.Nature. 1999 Sep 30;401(6752):485-9. doi: 10.1038/46802. Nature. 1999. PMID: 10519552
-
Import of carrier proteins into the mitochondrial inner membrane mediated by Tim22.Nature. 1996 Dec 12;384(6609):582-5. doi: 10.1038/384582a0. Nature. 1996. PMID: 8955274
-
Protein import into mitochondria.IUBMB Life. 2001 Sep-Nov;52(3-5):101-12. doi: 10.1080/15216540152845894. IUBMB Life. 2001. PMID: 11798021 Review.
-
Mitochondrial preprotein translocases as dynamic molecular machines.FEMS Yeast Res. 2006 Sep;6(6):849-61. doi: 10.1111/j.1567-1364.2006.00134.x. FEMS Yeast Res. 2006. PMID: 16911507 Review.
Cited by
-
Mechanisms of protein sorting in mitochondria.Cold Spring Harb Perspect Biol. 2012 Oct 1;4(10):a011320. doi: 10.1101/cshperspect.a011320. Cold Spring Harb Perspect Biol. 2012. PMID: 23028120 Free PMC article. Review.
-
A cold-stress-inducible PERK/OGT axis controls TOM70-assisted mitochondrial protein import and cristae formation.Cell Metab. 2021 Mar 2;33(3):598-614.e7. doi: 10.1016/j.cmet.2021.01.013. Epub 2021 Feb 15. Cell Metab. 2021. PMID: 33592173 Free PMC article.
-
Mitochondrial protein import stress.Nat Cell Biol. 2025 Feb;27(2):188-201. doi: 10.1038/s41556-024-01590-w. Epub 2025 Jan 22. Nat Cell Biol. 2025. PMID: 39843636 Review.
-
Function of cytosolic chaperones in Tom70-mediated mitochondrial import.Protein Pept Lett. 2011 Feb;18(2):122-31. doi: 10.2174/092986611794475020. Protein Pept Lett. 2011. PMID: 20955164 Free PMC article. Review.
-
Reduced Glucose Sensation Can Increase the Fitness of Saccharomyces cerevisiae Lacking Mitochondrial DNA.PLoS One. 2016 Jan 11;11(1):e0146511. doi: 10.1371/journal.pone.0146511. eCollection 2016. PLoS One. 2016. PMID: 26751567 Free PMC article.
References
-
- Alconada A., Gärtner,F., Hönlinger,A., Kübrich,M. and Pfanner,N. (1995) Mitochondrial receptor complex from Neurospora crassa and Saccharomyces cerevisiae. Methods Enzymol., 260, 263–286. - PubMed
-
- Bömer U., Pfanner,N. and Dietmeier,K. (1996) Identification of a third yeast mitochondrial Tom protein with tetratricopeptide repeats. FEBS Lett., 382, 153–158. - PubMed
-
- Brix J., Rüdiger,S., Bukau,B., Schneider-Mergener,J. and Pfanner,N. (1999) Distribution of binding sequences for the mitochondrial import receptors Tom20, Tom22 and Tom70 in a presequence-carrying preprotein and a non-cleavable preprotein. J. Biol. Chem., 274, 16522–16530. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

