Fission yeast Pom1p kinase activity is cell cycle regulated and essential for cellular symmetry during growth and division
- PMID: 11230130
- PMCID: PMC145493
- DOI: 10.1093/emboj/20.5.1064
Fission yeast Pom1p kinase activity is cell cycle regulated and essential for cellular symmetry during growth and division
Abstract
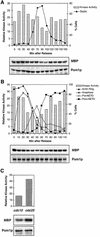
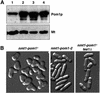
Schizosaccharomyces pombe cells grow from both ends during most of interphase and divide symmetrically into two daughter cells. The pom1 gene, encoding a member of the Dyrk family of protein kinases, has been identified through a mutant showing abnormal cellular morphogenesis. Here we show that Pom1p kinase activity is cell cycle regulated in correlation with the state of cellular symmetry: the activity is high during symmetrical growth and division, but lower when cells grow at just one end. Point mutations in the catalytic domain lead to asymmetry during both cell growth and division, whilst cells overexpressing Pom1p form additional growing ends. Manipulations of kinase activity indicate a negative role for Pom1p in microtubule growth at cell ends. Pom1p is present in a large protein complex and requires its non-catalytic domain to localize to the cell periphery and its kinase activity to localize to cell ends. These data establish that Pom1p kinase activity plays an important role in generating cellular symmetry and suggest that there may be related roles of homologous protein kinases ubiquitously present in all eukaryotes.
Figures





References
-
- Bähler J. and Peter,M. (2000) Cell polarity in yeast. In Drubin,D.G. (ed.), Cell Polarity. Oxford University Press, Oxford, UK, pp. 21–77.
-
- Bähler J., Wu,J.-Q., Longtine,M.S., Shah,N.G., McKenzie,A.M.,III, Steever,A.B., Wach,A., Philippsen,P. and Pringle,J.R. (1998b) Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast, 14, 943–951. - PubMed
-
- Becker W. and Joost,H.G. (1999) Structural and functional characteristics of Dyrk, a novel subfamily of protein kinases with dual specificity. Prog. Nucleic Acid Res. Mol. Biol., 62, 1–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

