The human cytomegalovirus immediate early 2 protein dissociates cellular DNA synthesis from cyclin-dependent kinase activation
- PMID: 11230132
- PMCID: PMC145458
- DOI: 10.1093/emboj/20.5.1086
The human cytomegalovirus immediate early 2 protein dissociates cellular DNA synthesis from cyclin-dependent kinase activation
Abstract
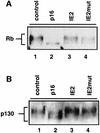
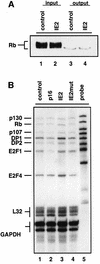
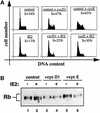
Passage through the restriction point late in G1 normally commits cells to replicate their DNA. Here we show that the previously reported cell cycle block mediated by the human cytomegalovirus (HCMV) immediate early 2 (IE2) protein uncouples this association. First, IE2 expression leads to elevated levels of cyclin E-associated kinase activity via transcriptional activation of the cyclin E gene. This contributes to post-restriction point characteristics of IE2-expressing cells. Then these cells fail to undergo substantial DNA replication although they have entered S phase, and the induction of DNA replication observed after overexpression of cyclin E or D can be antagonized by IE2 without impinging on cyclin-associated kinase activities. These data suggest that IE2 secures restriction-point transition of cells before it stops them from replicating their genome. Our results fit well with HCMV physiology and support the view that IE2 is part of a viral activity which, on the one hand, promotes cell cycle-dependent expression of cellular replication factors but, on the other hand, disallows competitive cellular DNA synthesis.
Figures






Similar articles
-
Dysregulation of cyclin E gene expression in human cytomegalovirus-infected cells requires viral early gene expression and is associated with changes in the Rb-related protein p130.J Virol. 2000 May;74(9):4192-206. doi: 10.1128/jvi.74.9.4192-4206.2000. J Virol. 2000. PMID: 10756032 Free PMC article.
-
Disruption of PML-associated nuclear bodies by IE1 correlates with efficient early stages of viral gene expression and DNA replication in human cytomegalovirus infection.Virology. 2000 Aug 15;274(1):39-55. doi: 10.1006/viro.2000.0448. Virology. 2000. PMID: 10936087
-
Recruitment of cdk9 to the immediate-early viral transcriptosomes during human cytomegalovirus infection requires efficient binding to cyclin T1, a threshold level of IE2 86, and active transcription.J Virol. 2009 Jun;83(11):5904-17. doi: 10.1128/JVI.02651-08. Epub 2009 Mar 18. J Virol. 2009. PMID: 19297489 Free PMC article.
-
Cell cycle dysregulation by human cytomegalovirus: influence of the cell cycle phase at the time of infection and effects on cyclin transcription.J Virol. 1998 May;72(5):3729-41. doi: 10.1128/JVI.72.5.3729-3741.1998. J Virol. 1998. PMID: 9557655 Free PMC article.
-
Human cytomegalovirus late protein encoded by ie2: a trans-activator as well as a repressor of gene expression.J Gen Virol. 1994 Sep;75 ( Pt 9):2337-48. doi: 10.1099/0022-1317-75-9-2337. J Gen Virol. 1994. PMID: 8077932
Cited by
-
Human cytomegalovirus riding the cell cycle.Med Microbiol Immunol. 2015 Jun;204(3):409-19. doi: 10.1007/s00430-015-0396-z. Epub 2015 Mar 17. Med Microbiol Immunol. 2015. PMID: 25776080 Review.
-
Antagonistic Relationship between Human Cytomegalovirus pUL27 and pUL97 Activities during Infection.J Virol. 2015 Oct;89(20):10230-46. doi: 10.1128/JVI.00986-15. Epub 2015 Jul 29. J Virol. 2015. PMID: 26223645 Free PMC article.
-
Exon 3 of the human cytomegalovirus major immediate-early region is required for efficient viral gene expression and for cellular cyclin modulation.J Virol. 2005 Jun;79(12):7438-52. doi: 10.1128/JVI.79.12.7438-7452.2005. J Virol. 2005. PMID: 15919900 Free PMC article.
-
Human cytomegalovirus tegument protein pp150 acts as a cyclin A2-CDK-dependent sensor of the host cell cycle and differentiation state.Proc Natl Acad Sci U S A. 2013 Oct 22;110(43):17510-5. doi: 10.1073/pnas.1312235110. Epub 2013 Oct 7. Proc Natl Acad Sci U S A. 2013. PMID: 24101496 Free PMC article.
-
Varicella-zoster virus infection of human foreskin fibroblast cells results in atypical cyclin expression and cyclin-dependent kinase activity.J Virol. 2006 Jun;80(11):5577-87. doi: 10.1128/JVI.00163-06. J Virol. 2006. PMID: 16699039 Free PMC article.
References
-
- Adams P.D., Lopez,P., Sellers,W.R. and Kaelin,W.G.,Jr (1997) Fluorescence-activated cell sorting of transfected cells. Methods Enzymol., 283, 59–72. - PubMed
-
- Agami R. and Bernards,R. (2000) Distinct initiation and maintenance mechanisms cooperate to induce G1 cell cycle arrest in response to DNA damage. Cell, 102, 55–66. - PubMed
-
- Anders D.G. and McCue,L.A. (1996) The human cytomegalovirus genes and proteins required for DNA synthesis. Intervirology, 39, 378–388. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

