Regulation of the Sko1 transcriptional repressor by the Hog1 MAP kinase in response to osmotic stress
- PMID: 11230135
- PMCID: PMC145460
- DOI: 10.1093/emboj/20.5.1123
Regulation of the Sko1 transcriptional repressor by the Hog1 MAP kinase in response to osmotic stress
Abstract
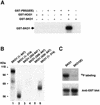
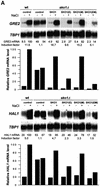
Exposure of yeast to increases in extracellular osmolarity activates the Hog1 mitogen-activated protein kinase (MAPK), which is essential for the induction of gene expression required for cell survival upon osmotic stress. Several genes are regulated in response to osmotic stress by Sko1, a transcriptional repressor of the ATF/CREB family. We show by in vivo coprecipitation and phosphorylation studies that Sko1 and Hog1 interact and that Sko1 is phosphorylated upon osmotic stress in a Hog1-dependent manner. Hog1 phosphorylates Sko1 in vitro at multiple sites within the N-terminal region. Phosphorylation of Sko1 disrupts the Sko1-Ssn6-Tup1 repressor complex, and consistently, a mutant allele of Sko1, unphosphorylatable by Hog1, exhibits less derepression than the wild type. Interestingly, Sko1 repressor activity is further enhanced in strains with high protein kinase A (PKA) activity. PKA phosphorylates Sko1 near the bZIP domain and mutation of these sites eliminates modulation of Sko1 responses to high PKA activity. Thus, Sko1 transcriptional repression is controlled directly by the Hog1 MAPK in response to stress, and this effect is further modulated by an independent signaling mechanism through the PKA pathway.
Figures

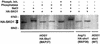








Similar articles
-
Hog1 kinase converts the Sko1-Cyc8-Tup1 repressor complex into an activator that recruits SAGA and SWI/SNF in response to osmotic stress.Mol Cell. 2002 Jun;9(6):1307-17. doi: 10.1016/s1097-2765(02)00557-9. Mol Cell. 2002. PMID: 12086627
-
Repressors and upstream repressing sequences of the stress-regulated ENA1 gene in Saccharomyces cerevisiae: bZIP protein Sko1p confers HOG-dependent osmotic regulation.Mol Cell Biol. 1999 Jan;19(1):537-46. doi: 10.1128/MCB.19.1.537. Mol Cell Biol. 1999. PMID: 9858577 Free PMC article.
-
Genomewide identification of Sko1 target promoters reveals a regulatory network that operates in response to osmotic stress in Saccharomyces cerevisiae.Eukaryot Cell. 2005 Aug;4(8):1343-52. doi: 10.1128/EC.4.8.1343-1352.2005. Eukaryot Cell. 2005. PMID: 16087739 Free PMC article.
-
Osmotic stress signaling and osmoadaptation in yeasts.Microbiol Mol Biol Rev. 2002 Jun;66(2):300-72. doi: 10.1128/MMBR.66.2.300-372.2002. Microbiol Mol Biol Rev. 2002. PMID: 12040128 Free PMC article. Review.
-
Multilayered control of gene expression by stress-activated protein kinases.EMBO J. 2010 Jan 6;29(1):4-13. doi: 10.1038/emboj.2009.346. Epub 2009 Nov 26. EMBO J. 2010. PMID: 19942851 Free PMC article. Review.
Cited by
-
Distinct regions of ATF/CREB proteins Atf1 and Pcr1 control recombination hotspot ade6-M26 and the osmotic stress response.Nucleic Acids Res. 2008 May;36(9):2838-51. doi: 10.1093/nar/gkn037. Epub 2008 Mar 29. Nucleic Acids Res. 2008. PMID: 18375981 Free PMC article.
-
Condition-specific genetic interaction maps reveal crosstalk between the cAMP/PKA and the HOG MAPK pathways in the activation of the general stress response.Mol Syst Biol. 2015 Oct 7;11(10):829. doi: 10.15252/msb.20156451. Mol Syst Biol. 2015. PMID: 26446933 Free PMC article.
-
Reduced TOR signaling sustains hyphal development in Candida albicans by lowering Hog1 basal activity.Mol Biol Cell. 2013 Feb;24(3):385-97. doi: 10.1091/mbc.E12-06-0477. Epub 2012 Nov 21. Mol Biol Cell. 2013. PMID: 23171549 Free PMC article.
-
Osmostress-induced gene expression--a model to understand how stress-activated protein kinases (SAPKs) regulate transcription.FEBS J. 2015 Sep;282(17):3275-85. doi: 10.1111/febs.13323. Epub 2015 Jun 10. FEBS J. 2015. PMID: 25996081 Free PMC article. Review.
-
Elongating under Stress.Genet Res Int. 2011;2011:326286. doi: 10.4061/2011/326286. Epub 2011 Sep 7. Genet Res Int. 2011. PMID: 22567351 Free PMC article.
References
-
- Boguslawski G. (1992) PBS2, a yeast gene encoding a putative protein kinase, interacts with the RAS2 pathway and affects osmotic sensitivity of Saccharomyces cerevisiae. J. Gen. Microbiol., 138, 2425–2432. - PubMed
-
- Brewster J.L., de Valoir,T., Dwyer,N.D., Winter,E. and Gustin,M.C. (1993) An osmosensing signal transduction pathway in yeast. Science, 259, 1760–1763. - PubMed
-
- Carlson M. and Botstein,D. (1982) Two differentially regulated mRNAs with different 5′-ends encode secreted and intracellular forms of yeast invertase. Cell, 28, 145–154. - PubMed
-
- DeVit M.J. and Johnston,M. (1999) The nuclear exportin Msn5 is required for nuclear export of the Mig1 glucose repressor of Saccharomyces cerevisiae. Curr. Biol., 9, 1231–1241. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

