Ten1 functions in telomere end protection and length regulation in association with Stn1 and Cdc13
- PMID: 11230140
- PMCID: PMC145504
- DOI: 10.1093/emboj/20.5.1173
Ten1 functions in telomere end protection and length regulation in association with Stn1 and Cdc13
Abstract
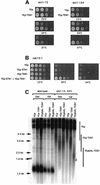
In Saccharomyces cerevisiae, Cdc13 has been proposed to mediate telomerase recruitment at telomere ends. Stn1, which associates with Cdc13 by the two-hybrid interaction, has been implicated in telomere maintenance. Ten1, a previously uncharacterized protein, was found to associate physically with both Stn1 and Cdc13. A binding defect between Stn1-13 and Ten1 was responsible for the long telomere phenotype of stn1-13 mutant cells. Moreover, rescue of the cdc13-1 mutation by STN1 was much improved when TEN1 was simultaneously overexpressed. Several ten1 mutations were found to confer telomerase-dependent telomere lengthening. Other, temperature-sensitive, mutants of TEN1 arrested at G(2)/M via activation of the Rad9-dependent DNA damage checkpoint. These ten1 mutant cells were found to accumulate single-stranded DNA in telomeric regions of the chromosomes. We propose that Ten1 is required to regulate telomere length, as well as to prevent lethal damage to telomeric DNA.
Figures




Similar articles
-
Dysfunction of Telomeric Cdc13-Stn1-Ten1 Simultaneously Activates DNA Damage and Spindle Checkpoints.Cells. 2024 Sep 25;13(19):1605. doi: 10.3390/cells13191605. Cells. 2024. PMID: 39404369 Free PMC article.
-
TEN1 is essential for CDC13-mediated telomere capping.Genetics. 2009 Nov;183(3):793-810. doi: 10.1534/genetics.109.108894. Epub 2009 Sep 14. Genetics. 2009. PMID: 19752213 Free PMC article.
-
Cdc13 cooperates with the yeast Ku proteins and Stn1 to regulate telomerase recruitment.Mol Cell Biol. 2000 Nov;20(22):8397-408. doi: 10.1128/MCB.20.22.8397-8408.2000. Mol Cell Biol. 2000. PMID: 11046137 Free PMC article.
-
Evolution of CST function in telomere maintenance.Cell Cycle. 2010 Aug 15;9(16):3157-65. doi: 10.4161/cc.9.16.12547. Epub 2010 Aug 26. Cell Cycle. 2010. PMID: 20697207 Free PMC article. Review.
-
CST meets shelterin to keep telomeres in check.Mol Cell. 2010 Sep 10;39(5):665-76. doi: 10.1016/j.molcel.2010.08.024. Mol Cell. 2010. PMID: 20832719 Review.
Cited by
-
Telomere C-Strand Fill-In Machinery: New Insights into the Human CST-DNA Polymerase Alpha-Primase Structures and Functions.Subcell Biochem. 2024;104:73-100. doi: 10.1007/978-3-031-58843-3_5. Subcell Biochem. 2024. PMID: 38963484 Review.
-
Intrachromatid excision of telomeric DNA as a mechanism for telomere size control in Saccharomyces cerevisiae.Mol Cell Biol. 2001 Oct;21(19):6559-73. doi: 10.1128/MCB.21.19.6559-6573.2001. Mol Cell Biol. 2001. PMID: 11533244 Free PMC article.
-
Murine Pif1 interacts with telomerase and is dispensable for telomere function in vivo.Mol Cell Biol. 2007 Feb;27(3):1017-26. doi: 10.1128/MCB.01866-06. Epub 2006 Nov 27. Mol Cell Biol. 2007. PMID: 17130244 Free PMC article.
-
Telomere capping proteins are structurally related to RPA with an additional telomere-specific domain.Proc Natl Acad Sci U S A. 2009 Nov 17;106(46):19298-303. doi: 10.1073/pnas.0909203106. Epub 2009 Nov 2. Proc Natl Acad Sci U S A. 2009. PMID: 19884503 Free PMC article.
-
Genetic analysis reveals essential and non-essential amino acids within the telomeric DNA-binding interface of Cdc13p.Biochem J. 2007 Apr 15;403(2):289-95. doi: 10.1042/BJ20061698. Biochem J. 2007. PMID: 17166094 Free PMC article.
References
-
- Ausubel F.A., Brent,R., Kingston,R.E., Moore,D.D., Seidman,J.G., Smith,J.A. and Struhl,K. (eds) (1998) Current Protocols in Molecular Biology. John Wiley and Sons, New York, NY.
-
- Bertuch A. and Lundblad,V. (1998) Telomeres and double-strand breaks: trying to make the ends meet. Trends Cell Biol., 8, 339–342. - PubMed
-
- Diede S.J. and Gottschling,D.E. (1999) Telomerase-mediated telomere addition in vivo requires DNA primase and DNA polymerases α and δ. Cell, 99, 723–733. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

