A model for the abrogation of the SOS response by an SOS protein: a negatively charged helix in DinI mimics DNA in its interaction with RecA
- PMID: 11230150
- PMCID: PMC312637
- DOI: 10.1101/gad.862901
A model for the abrogation of the SOS response by an SOS protein: a negatively charged helix in DinI mimics DNA in its interaction with RecA
Abstract
DinI is a recently described negative regulator of the SOS response in Escherichia coli. Here we show that it physically interacts with RecA and prevents the binding of single-stranded DNA to RecA, which is required for the activation of the latter. DinI also displaces ssDNA from a stable RecA-DNA cofilament, thus eliminating the SOS signal. In addition, DinI inhibits RecA-mediated homologous DNA pairing, but has no effect on actively proceeding strand exchange. Biochemical data, together with the molecular structure, define the C-terminal alpha-helix in DinI as the active site of the protein. In an unusual example of molecular mimicry, a negatively charged surface on this alpha-helix, by imitating single-stranded DNA, interacts with the loop L2 homologous pairing region of RecA and interferes with the activation of RecA.
Figures





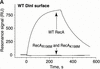





References
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, Albright LM, Coen DM, Varki A. Current protocols in molecular biology. New York: John Wiley; 1998.
-
- Bax A, Grzesiek S. Methodological advances in protein NMR. Accounts Chem Res. 1993;26:131–138.
-
- Bebenek A, Pietrzykowska I. A new mutation in Escherichia coli K12, isfA, which is responsible for inhibition of SOS functions. Mol Gen Genet. 1995;248:103–113. - PubMed
-
- ————— The isfA mutation inhibits mutator activity and processing of UmuD protein in Escherichia coli recA730 strains. Mol Gen Genet. 1996;250:674–680. - PubMed
-
- Bianco PR, Tracy RB, Kowalczykowski SC. DNA strand exchange proteins: A biochemical and physical comparison. Front Biosci. 1998;3:D570–D603. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
