Development of a species-specific PCR assay for detection of Leishmania donovani in clinical samples from patients with kala-azar and post-kala-azar dermal leishmaniasis
- PMID: 11230394
- PMCID: PMC87840
- DOI: 10.1128/JCM.39.3.849-854.2001
Development of a species-specific PCR assay for detection of Leishmania donovani in clinical samples from patients with kala-azar and post-kala-azar dermal leishmaniasis
Abstract
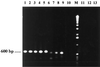
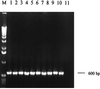
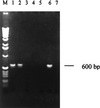
We have developed a PCR assay that is capable of amplifying kinetoplast DNA (kDNA) of Leishmania donovani in a species-specific manner among Old World leishmanias. With Indian strains and isolates of L. donovani the assay was sensitive enough to detect kDNA in an amount equivalent to a single parasite or less. The extreme sensitivity of the assay was reflected in its ability to detect parasite DNA from small volumes of peripheral blood of patients with kala-azar (KA) and from skin lesions from patients with post-KA dermal leishmaniasis (PKDL). A total of 107 clinical leishmaniasis samples were analyzed. Of these 102 (95.3%) were positive by PCR. The test provided a diagnosis of KA with 96% sensitivity using patient whole-blood samples instead of bone marrow or spleen aspirates that are obtained by invasive procedures. The assay was also successful in the diagnosis of 45 of 48 PKDL cases (93.8%). Cross-reactions with pathogens prevalent in the area of endemicity, viz., Mycobacterium tuberculosis, Mycobacterium leprae, and Plasmodium spp., could be ruled out. Eighty-one control samples, including dermal scrapings from healthy portions of skin from patients with PKDL were all negative. Two of twenty controls from the area of endemicity were found positive by PCR assay; however, there was a good possibility that these two were asymptomatic carriers since they were serologically positive for KA. Thus, this PCR assay represents a tool for the diagnosis of KA and PKDL in Indian patients in a noninvasive manner, with simultaneous species identification of parasites in clinical samples.
Figures



References
-
- Adhya S, Chatterjee M, Hassan M Q, Mukherjee S, Sen S. Detection of Leishmania in the blood of early kala-azar patients with the aid of polymerase chain reaction. Trans R Soc Trop Med Hyg. 1995;89:622–624. - PubMed
-
- Albrecht H, Sobottka I, Emminger C, Jablonowski H, Just G, Stoehr A, Kubin T, Salzberger B, Lutz T, Lunzen J V. Leishmaniasis and HIV. Arch Pathol Lab Med. 1996;120:189–198. - PubMed
-
- Andresen K, Gaafar A, El Hassan A M, Ismail A, Dafalla M, Theander T G, Kharazmi A. Evaluation of the polymerase chain reaction in the diagnosis of cutaneous leishmaniasis due to Leishmania major. A comparison with direct microscopy of smears and sections from lesions. Trans R Soc Trop Med Hyg. 1996;90:133–135. - PubMed
-
- Andresen K, Gasim S, El Hassan A M, Khalil E A, Barker D C, Theander T G, Kharazmi A. Diagnosis of visceral leishmaniasis by polymerase chain reaction using blood, bone marrow and lymph node samples from patients from the Sudan. Trop Med Int Health. 1997;2:440–444. - PubMed
-
- Aviles H, Belli A, Armijos R, Monroy F P, Harris E. PCR detection and identification of Leishmania parasites in clinical specimens in Ecuador: a comparison with classical diagnostic methods. J Parasitol. 1999;85:181–187. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

