Mannosidase production by viridans group streptococci
- PMID: 11230417
- PMCID: PMC87863
- DOI: 10.1128/JCM.39.3.995-1001.2001
Mannosidase production by viridans group streptococci
Abstract
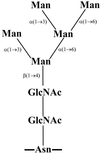
The production of mannosidase activity by all currently recognized species of human viridans group streptococci was determined using an assay in which bacterial growth was dependent on the degradation of the high-mannose-type glycans of RNase B and subsequent utilization of released mannose. RNase B is an excellent substrate for the demonstration of mannosidase activity since it is a glycoprotein with a single glycosylation site which is occupied by high-mannose-type glycoforms containing five to nine mannose residues. Mannosidase activity was produced only by some members of the mitis group (Streptococcus mitis, Streptococcus oralis, Streptococcus gordonii, Streptococcus cristatus, Streptococcus infantis, Streptococcus parasanguinis, and Streptococcus pneumoniae) and Streptococcus intermedius of the anginosus group. None of the other species within the salivarius and mutans groups or Streptococcus peroris and Streptococcus sanguinis produced mannosidase activity. Using matrix-assisted laser desorption ionization time-of-flight mass spectrometry, it was demonstrated that the Man(5) glycan alone was degraded while Man(6) to Man(9), which contain terminal alpha(1-->2) mannose residues in addition to the alpha(1-->3), alpha(1-->6), and beta(1-->4) residues present in Man(5), remained intact. Investigations on mannosidase production using synthetic (4-methylumbelliferone- or p-nitrophenol-linked) alpha- or beta-mannosides as substrates indicated that there was no correlation between degradation of these substrates and degradation of the Man(5) glycan of RNase B. No species degraded these alpha-linked mannosides, while degradation of the beta-linked synthetic substrates was restricted to strains within the Streptococcus anginosus, S. gordonii, and S. intermedius species. The data generated using a native glycoprotein as the substrate demonstrate that mannosidase production within the viridans group streptococci is more widely distributed than had previously been considered.
Figures

Similar articles
-
Growth of Viridans streptococci on human serum alpha1-acid glycoprotein.J Dent Res. 1999 Jul;78(7):1370-80. doi: 10.1177/00220345990780071201. J Dent Res. 1999. PMID: 10403465
-
Detecting mannosidase activities using ribonuclease B and matrix-assisted laser desorption/ionization-time of flight mass spectrometry.Anal Biochem. 2000 Jul 1;282(2):165-72. doi: 10.1006/abio.2000.4606. Anal Biochem. 2000. PMID: 10873270
-
Differentiation of mutans streptococci by intact cell matrix-assisted laser desorption/ionization time-of-flight mass spectrometry.Oral Microbiol Immunol. 2005 Oct;20(5):267-73. doi: 10.1111/j.1399-302X.2005.00223.x. Oral Microbiol Immunol. 2005. PMID: 16101961
-
Production of an endo-beta-N-acetylglucosaminidase activity mediates growth of Enterococcus faecalis on a high-mannose-type glycoprotein.J Bacteriol. 2000 Feb;182(4):882-90. doi: 10.1128/JB.182.4.882-890.2000. J Bacteriol. 2000. PMID: 10648510 Free PMC article.
-
[Classical and new approaches in laboratory diagnosis of viridans streptococci].Mikrobiyol Bul. 2010 Jul;44(3):495-503. Mikrobiyol Bul. 2010. PMID: 21064001 Review. Turkish.
Cited by
-
Growth of Streptococcus pneumoniae on human glycoconjugates is dependent upon the sequential activity of bacterial exoglycosidases.J Bacteriol. 2008 Jan;190(1):221-30. doi: 10.1128/JB.01251-07. Epub 2007 Nov 2. J Bacteriol. 2008. PMID: 17981977 Free PMC article.
-
Biofilm mode of growth of Streptococcus intermedius favored by a competence-stimulating signaling peptide.J Bacteriol. 2004 Sep;186(18):6327-31. doi: 10.1128/JB.186.18.6327-6331.2004. J Bacteriol. 2004. PMID: 15342606 Free PMC article.
-
Role of UDP-N-acetylglucosamine2-epimerase/N-acetylmannosamine kinase (GNE) in β1-integrin-mediated cell adhesion.Mol Neurobiol. 2014 Oct;50(2):257-73. doi: 10.1007/s12035-013-8604-6. Epub 2014 Jan 29. Mol Neurobiol. 2014. PMID: 24474513
-
Impact of bacterial vaginosis, as assessed by nugent criteria and hormonal status on glycosidases and lectin binding in cervicovaginal lavage samples.PLoS One. 2015 May 26;10(5):e0127091. doi: 10.1371/journal.pone.0127091. eCollection 2015. PLoS One. 2015. PMID: 26011704 Free PMC article.
-
The Effects of Hormones and Vaginal Microflora on the Glycome of the Female Genital Tract: Cervical-Vaginal Fluid.PLoS One. 2016 Jul 20;11(7):e0158687. doi: 10.1371/journal.pone.0158687. eCollection 2016. PLoS One. 2016. PMID: 27437931 Free PMC article.
References
-
- Beighton D, Carr A D, Oppenheim B A. Identification of viridans streptococci associated with bacteraemia in neutropenic cancer patients. J Med Microbiol. 1994;40:202–204. - PubMed
-
- Beighton D, Hardie J M, Whiley R A. A scheme for the identification of viridans streptococci. J Med Microbiol. 1991;35:367–372. - PubMed
-
- Bochud P-Y, Calandra T, Francioli P. Bacteremia due to viridans streptococci in neutropenic patients: a review. Am J Med. 1994;97:256–264. - PubMed
-
- Byers H L, Tarelli E, Homer K A, Beighton D. Sequential deglycosylation and utilization of the N-linked, complex-type glycans of human α1-acid glycoprotein mediates growth of Streptococcus oralis. Glycobiology. 1999;9:469–479. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

