Persistent ICT malaria P.f/P.v panmalarial and HRP2 antigen reactivity after treatment of Plasmodium falciparum malaria is associated with gametocytemia and results in false-positive diagnoses of Plasmodium vivax in convalescence
- PMID: 11230422
- PMCID: PMC87868
- DOI: 10.1128/JCM.39.3.1025-1031.2001
Persistent ICT malaria P.f/P.v panmalarial and HRP2 antigen reactivity after treatment of Plasmodium falciparum malaria is associated with gametocytemia and results in false-positive diagnoses of Plasmodium vivax in convalescence
Abstract
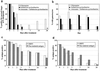
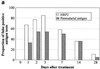
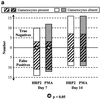
A problem with rapid Plasmodium falciparum-specific antigen histidine-rich protein 2 (HRP2) detection tests for malaria is the persistence of antigen in blood after the disappearance of asexual-stage parasitemia and clinical symptoms, resulting in false-positive (FP) test results following treatment. The ICT P.f/P.v immunochromatographic test detects both HRP2 and a panmalarial antigen (PMA) found in both P. falciparum and Plasmodium vivax. To examine posttreatment antigen persistence with this test and whether persistent sexual-stage forms (gametocytes) are a cause of FP tests after treatment, we compared serial antigen test results with microscopy results from patients symptomatic with P. falciparum malaria in Indonesia for 28 days following treatment with chloroquine (CQ; n = 66), sulfadoxine-pyrimethamine (SP; n = 36), and artesunate plus sulfadoxine-pyrimethamine (ART + SP; n = 15). Persistent FP antigenemia following SP treatment occurred in 29% (HRP2) and 42% (PMA) of the patients on day 7 and in 10% (HRP2) and 23% (PMA) on day 14. The high rates of persistent HRP2 and PMA antigenemia following CQ and SP treatment were strongly associated with the presence of gametocytemia, with the proportion with gametocytes on day 7 posttreatment being significantly greater in those with FP results than in those with true-negative PMA and HRP2 results. Gametocyte frequency on day 14 post-SP treatment was also greater in those with FP PMA results. Following SP treatment, PMA persisted longer than HRP2, giving an FP diagnosis of P. vivax in up to 16% of patients on day 14, with all FP P. vivax diagnoses having gametocytemia. In contrast, PMA was rapidly cleared following ART + SP treatment in association with rapid clearance of gametocytemia. Gametocytes appear to be an important cause of persistent posttreatment panmalarial antigenemia in areas of endemicity and may also contribute in part to persistent HRP2 antigenemia following treatment.
Figures




Similar articles
-
Laboratory evaluation of the ict malaria P.f./P.v. immunochromatographic test for detecting the panmalarial antigen using a rodent malaria model.Am J Trop Med Hyg. 2004 Feb;70(2):139-43. Am J Trop Med Hyg. 2004. PMID: 14993624
-
Persistent histidine-rich protein 2, parasite lactate dehydrogenase, and panmalarial antigen reactivity after clearance of Plasmodium falciparum monoinfection.J Clin Microbiol. 2004 Sep;42(9):4237-41. doi: 10.1128/JCM.42.9.4237-4241.2004. J Clin Microbiol. 2004. PMID: 15365017 Free PMC article.
-
Rapid immunochromatography-based detection of mixed-species malaria infection in Pakistan.Southeast Asian J Trop Med Public Health. 2005 May;36(3):562-4. Southeast Asian J Trop Med Public Health. 2005. PMID: 16124417
-
Overview: immunology of malaria and progress in malaria vaccine development.Southeast Asian J Trop Med Public Health. 1992 Sep;23 Suppl 4:71-87. Southeast Asian J Trop Med Public Health. 1992. PMID: 1364871 Review. No abstract available.
-
Immunity against sexual stage Plasmodium falciparum and Plasmodium vivax parasites.Immunol Rev. 2020 Jan;293(1):190-215. doi: 10.1111/imr.12828. Epub 2019 Dec 16. Immunol Rev. 2020. PMID: 31840844 Free PMC article. Review.
Cited by
-
Performance of rapid diagnostic test, light microscopy, and polymerase chain reaction in pregnant women with asymptomatic malaria in Nigeria.IJID Reg. 2024 Aug 2;12:100416. doi: 10.1016/j.ijregi.2024.100416. eCollection 2024 Sep. IJID Reg. 2024. PMID: 39253688 Free PMC article.
-
Quantitative detection of PfHRP2 in saliva of malaria patients in the Philippines.Malar J. 2012 May 25;11:175. doi: 10.1186/1475-2875-11-175. Malar J. 2012. PMID: 22631858 Free PMC article.
-
Low Prevalence of Deletions of the pfhrp2 and pfhrp3 Genes in Plasmodium falciparum Parasites in Freetown, Sierra Leone in 2015.Am J Trop Med Hyg. 2022 Jun 15;106(6):1667-1669. doi: 10.4269/ajtmh.22-0073. Print 2022 Jun 15. Am J Trop Med Hyg. 2022. PMID: 35895430 Free PMC article.
-
Plasmodium falciparum histidine-rich protein (PfHRP2 and 3) diversity in Western and Coastal Kenya.Sci Rep. 2019 Feb 8;9(1):1709. doi: 10.1038/s41598-018-38175-1. Sci Rep. 2019. PMID: 30737461 Free PMC article.
-
Evaluation of a PfHRP2 and a pLDH-based rapid diagnostic test for the diagnosis of severe malaria in 2 populations of African children.Clin Infect Dis. 2011 May;52(9):1100-7. doi: 10.1093/cid/cir143. Clin Infect Dis. 2011. PMID: 21467015 Free PMC article.
References
-
- Banchongaksorn T, Yomokgul P, Panyim S, Rooney W, Vickers P. A field trial of the ParaSight-F test for the diagnosis of Plasmodium falciparum infection. Trans R Soc Trop Med Hyg. 1996;90:244–245. - PubMed
-
- Beadle C, Long G, Weiss W, McElroy P, Maret S, Oloo A, Hoffman S. Diagnosis of malaria by detection of Plasmodium falciparum HRP-2 antigen with a rapid dipstick antigen-capture assay. Lancet. 1994;343:564–568. - PubMed
-
- Dean A G, Dean J A, Coulombier D, Brendel K A, Smith D C, Burton A H, Dicker R C, Sullivan K, Fagan R F, Arner T G. Epi Info, version 6: a word-processing database and statistics program for public health on IBM-compatible microcomputers. Atlanta, Ga: Centers for Disease Control and Prevention; 1995.
-
- Di Perri G, Olliaro P, Nardi S, Allegranzi B, Deganello R, Vento S, Lanzafame M, Cazzadori A, Bonora S, Concia E. The ParaSight-F rapid dipstick antigen capture assay for monitoring parasite clearance after drug treatment of Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 1997;91:403–405. - PubMed
-
- Dyer M E, Tjitra E, Currie B J, Anstey N M. Failure of the ‘pan-malarial’ antibody of the ICT Malaria P.f/P.v immunochromatographic test to detect symptomatic Plasmodium malariae infection. Trans R Soc Trop Med Hyg. 2000;94:518. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

