Transcranial magnetic stimulation and stretch reflexes in the tibialis anterior muscle during human walking
- PMID: 11230526
- PMCID: PMC2278473
- DOI: 10.1111/j.1469-7793.2001.0545i.x
Transcranial magnetic stimulation and stretch reflexes in the tibialis anterior muscle during human walking
Abstract
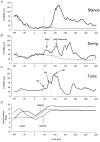
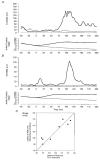
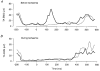
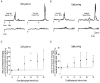
Stretch of the ankle dorsiflexors was applied at different times of the walking cycle in 17 human subjects. When the stretch was applied in the swing phase, only small and variable reflex responses were observed in the active tibialis anterior (TA) muscle. Two of the reflex responses that could be distinguished had latencies which were comparable with the early (M1) and late (M3)components of the three reflex responses (M1, M2 and M3) observed during tonic dorsiflexion in sitting subjects. In the stance phase a single very large response was consistently observed in the inactive TA muscle. The peak of this response had the same latency as the peak of M3, but in the majority of subjects the onset latency was shorter than that of M3. The TA reflex response in the stance phase was abolished by ischaemia of the lower leg at the same time as the soleus H-reflex, suggesting that large muscle afferents were involved in the generation of the response. Motor-evoked potentials (MEPs) elicited in the TA by transcranial magnetic stimulation (TMS) were strongly facilitated corresponding to the peak of the stretch response in the stance phase and the late reflex response in the swing phase. A similar facilitation was not observed corresponding to the earlier responses in the swing phase and the initial part of the response in stance. Prior stretch did not facilitate MEPs evoked by transcranial electrical stimulation in the swing phase of walking. However, in the stance phase MEPs elicited by strong electrical stimulation were facilitated by prior stretch to the same extent as the MEPs evoked by TMS. The large responses to stretch seen in the stance phase are consistent with the idea that stretch reflexes are mainly involved in securing the stability of the supporting leg during walking. It is suggested that a transcortical reflex pathway may be partly involved in the generation of the TA stretch responses during walking.
Figures

References
-
- Andersen JB, Sinkjaer T. An actuator system for investigating electrophysiological and biomechanical features around the human ankle joint during gait. Transaction on Rehabilitation Engineering. 1995;3:299–306.
-
- Andersson O, Forssberg H, Grillner S, Wallen P. Peripheral feedback mechanisms acting on the central pattern generators for locomotion in fish and cat. Canadian The Journal of Physiology and Pharmacology. 1981;59:713–26. - PubMed
-
- Capaday C, Lavoie BA, Barbeau H, Schneider C, Bonnard M. Studies on the corticospinal control of human walking. I. Responses to focal transcranial magnetic stimulation of the motor cortex. Journal of Neurophysiology. 1999;81:129–139. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

