Activation of a tissue-specific stress response in the aqueous outflow pathway of the eye defines the glaucoma disease phenotype
- PMID: 11231628
- PMCID: PMC1945815
- DOI: 10.1038/85446
Activation of a tissue-specific stress response in the aqueous outflow pathway of the eye defines the glaucoma disease phenotype
Abstract
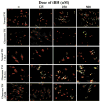
The glaucomas are a group of optic neuropathies comprising the leading cause of irreversible blindness worldwide. Elevated intraocular pressure due to a reduction in normal aqueous outflow is a major causal risk factor. We found that endothelial leukocyte adhesion molecule-1 (ELAM-1), the earliest marker for the atherosclerotic plaque in the vasculature, was consistently present on trabecular meshwork (TM) cells in the outflow pathways of eyes with glaucomas of diverse etiology. We determined expression of ELAM-1 to be controlled by activation of an interleukin-1 (IL-1) autocrine feedback loop through transcription factor NF-kappaB, and activity of this signaling pathway was shown to protect TM cells against oxidative stress. These findings characterize a protective stress response specific to the eye's aqueous outflow pathways and provide the first known diagnostic indicator of glaucomatous TM cells. They further indicate that common mechanisms contribute to the pathophysiology of the glaucomas and vascular diseases.
Figures



Comment in
-
Eyeing a new route along an old pathway.Nat Med. 2001 Mar;7(3):294-5. doi: 10.1038/85432. Nat Med. 2001. PMID: 11231625 No abstract available.
Similar articles
-
Glaucomatous MYOC mutations activate the IL-1/NF-κB inflammatory stress response and the glaucoma marker SELE in trabecular meshwork cells.Mol Vis. 2015 Sep 17;21:1071-84. eCollection 2015. Mol Vis. 2015. PMID: 26396484 Free PMC article.
-
Ultrasound activates the TM ELAM-1/IL-1/NF-kappaB response: a potential mechanism for intraocular pressure reduction after phacoemulsification.Invest Ophthalmol Vis Sci. 2003 May;44(5):1977-81. doi: 10.1167/iovs.02-0631. Invest Ophthalmol Vis Sci. 2003. PMID: 12714632 Free PMC article.
-
Expression of endothelial leukocyte adhesion molecule 1 in the aqueous outflow pathway of porcine eyes with induced glaucoma.Mol Vis. 2006 Dec 2;12:1467-72. Mol Vis. 2006. PMID: 17167401
-
Biomarkers and special features of oxidative stress in the anterior segment of the eye linked to lens cataract and the trabecular meshwork injury in primary open-angle glaucoma: challenges of dual combination therapy with N-acetylcarnosine lubricant eye drops and oral formulation of nonhydrolyzed carnosine.Fundam Clin Pharmacol. 2012 Feb;26(1):86-117. doi: 10.1111/j.1472-8206.2011.00969.x. Epub 2011 Aug 24. Fundam Clin Pharmacol. 2012. PMID: 21883446 Review.
-
The role of oxidative stress in glaucoma.Mutat Res. 2006 Mar;612(2):105-14. doi: 10.1016/j.mrrev.2005.11.001. Epub 2006 Jan 18. Mutat Res. 2006. PMID: 16413223 Review.
Cited by
-
Cellular and molecular biology of optineurin.Int Rev Cell Mol Biol. 2012;294:223-58. doi: 10.1016/B978-0-12-394305-7.00005-7. Int Rev Cell Mol Biol. 2012. PMID: 22364875 Free PMC article. Review.
-
Oxidative stress impact on barrier function of porcine angular aqueous plexus cell monolayers.Invest Ophthalmol Vis Sci. 2013 Jul 18;54(7):4827-35. doi: 10.1167/iovs.12-11435. Invest Ophthalmol Vis Sci. 2013. PMID: 23761078 Free PMC article.
-
Steroid-induced ocular hypertension/glaucoma: Focus on pharmacogenomics and implications for precision medicine.Prog Retin Eye Res. 2017 Jan;56:58-83. doi: 10.1016/j.preteyeres.2016.09.003. Epub 2016 Sep 22. Prog Retin Eye Res. 2017. PMID: 27666015 Free PMC article. Review.
-
Neuroinflammation in Primary Open-Angle Glaucoma.J Clin Med. 2020 Sep 30;9(10):3172. doi: 10.3390/jcm9103172. J Clin Med. 2020. PMID: 33007927 Free PMC article. Review.
-
Identification of a Novel Mucin Gene HCG22 Associated With Steroid-Induced Ocular Hypertension.Invest Ophthalmol Vis Sci. 2015 Apr;56(4):2737-48. doi: 10.1167/iovs.14-14803. Invest Ophthalmol Vis Sci. 2015. PMID: 25813999 Free PMC article.
References
-
- Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963;69:783–801. - PubMed
-
- Epstein DL, Allingham RR, Schuman JS, editors. Chandler and Grant’s Glaucoma. 4. Williams and Wilkins; Baltimore, Maryland: 1996. pp. 18–24.
-
- Underwood JL, et al. Glucocorticoids regulate transendothelial fluid flow resistance and formation of intracellular junctions. Am J Physiol. 1999;277:C330–342. - PubMed
-
- Tripathi RC. Mechanism of the aqueous outflow across the trabecular wall of Schlemm’s canal. Exp Eye Res. 1971;11:116–121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

