Cloning of dimethylglycine dehydrogenase and a new human inborn error of metabolism, dimethylglycine dehydrogenase deficiency
- PMID: 11231903
- PMCID: PMC1275637
- DOI: 10.1086/319520
Cloning of dimethylglycine dehydrogenase and a new human inborn error of metabolism, dimethylglycine dehydrogenase deficiency
Abstract
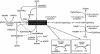
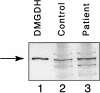
Dimethylglycine dehydrogenase (DMGDH) (E.C. number 1.5.99.2) is a mitochondrial matrix enzyme involved in the metabolism of choline, converting dimethylglycine to sarcosine. Sarcosine is then transformed to glycine by sarcosine dehydrogenase (E.C. number 1.5.99.1). Both enzymes use flavin adenine dinucleotide and folate in their reaction mechanisms. We have identified a 38-year-old man who has a lifelong condition of fishlike body odor and chronic muscle fatigue, accompanied by elevated levels of the muscle form of creatine kinase in serum. Biochemical analysis of the patient's serum and urine, using (1)H-nuclear magnetic resonance NMR spectroscopy, revealed that his levels of dimethylglycine were much higher than control values. The cDNA and the genomic DNA for human DMGDH (hDMGDH) were then cloned, and a homozygous A-->G substitution (326 A-->G) was identified in both the cDNA and genomic DNA of the patient. This mutation changes a His to an Arg (H109R). Expression analysis of the mutant cDNA indicates that this mutation inactivates the enzyme. We therefore confirm that the patient described here represents the first reported case of a new inborn error of metabolism, DMGDH deficiency.
Figures


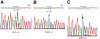

References
-
- Allen RH, Stabler SP, Lindenbaum J (1993) Serum betaine, N, N-dimethylglycine and N-methylglycine levels in patients with cobalamin and folate deficiency and related inborn errors of metabolism. Metabolism 42:1448–1460 - PubMed
-
- Bergeron F, Otto A, Blache P, Day R, Denoroy L, Brandsch R, Bataille D (1998) Molecular cloning and tissue distribution of rat sarcosine dehydrogenase. Eur J Biochem 257:556–561 - PubMed
-
- Binzak B, Willard J, Vockley J (1998) Identification of the catalytic residue of human short/branched chain acyl-CoA dehydrogenase by in vitro mutagenesis. Biochim Biophys Acta 1382:137–142 - PubMed
-
- Binzak BA, Vockley JG, Jenkins RB, Vockley J (2000) Structure and analysis of the human dimethylglycine dehydrogenase. Mol Genet Metab 69:181–187 - PubMed
-
- Cook RJ, Misono KS, Wagner C (1984) Identification of the covalently bound flavin of dimethylglycine dehydrogenase and sarcosine dehydrogenase from rat liver mitochondria. J Biol Chem 259:12475–12480 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

