Experimental MR imaging-guided interstitial cryotherapy of the brain
- PMID: 11237963
- PMCID: PMC7976821
Experimental MR imaging-guided interstitial cryotherapy of the brain
Abstract
Background and purpose: Hyperthermal ablation techniques such as laser or RF ablation require dedicated heat-sensitive MR imaging sequences for monitoring MR imaging--guided interventions. Because cryotherapy does not have these limitations, the purpose of this study was to evaluate the feasibility of MR imaging--guided percutaneous cryotherapy of the brain.
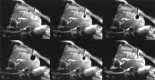
Methods: An experimental cryoprobe with an outer diameter of 2.7 mm was inserted into the right frontal lobe of 11 healthy pigs under MR imaging control. Freezing procedures were monitored by using an interventional 1.5-T magnet and a gradient-echo sequence with radial k-space trajectories, a fast T2-weighted single-shot spin-echo sequence, and a T1-weighted single-shot gradient-echo sequence. In three animals, the procedure was also monitored by using dynamic CT. A freeze-thaw cycle with a duration of 3 minutes was repeated three times per animal. Follow-up MR images were obtained 3, 7, and 14 days after cryotherapy by using conventional MR sequences. Six animals were killed 7 days after intervention, and five animals were killed 14 days after intervention. The brains were sectioned, and the histologic findings of the lesions were compared with the MR imaging appearance.
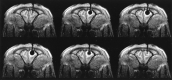
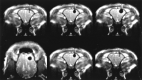
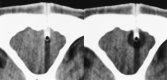
Results: No artifacts due to the probe were observed on the MR images or CT scans. The ice formation (mean diameter, 12.5 mm) was very well delineated as a signal-free sphere. MR monitoring of the freezing procedure yielded a significantly higher ice:tissue contrast than did CT. The size of the ice ball as imaged by MR imaging and CT during the intervention correlated well with the MR imaging appearance of the lesions at the 14-day follow-up examination and with the histologic findings. Histologically, coagulation necrosis and gliosis were found, surrounded by a transition zone of edema and a disrupted blood-brain barrier, corresponding to a contrast-enhancing rim around the lesions on follow-up MR images.
Conclusion: MR imaging-guided cryotherapy of the brain is possible and allows a precise prediction of the resulting necrosis. MR imaging of the freezing process does not require heat-sensitive sequences and is superior to CT for monitoring of cryoablation.
Figures


References
-
- Bernays R, Kollias S, Romanowski BJ. Real-time MR-guided neurosurgical interventions. In: Debatin JF, Adam G, eds. Interventional Magnetic Resonance Imaging. Berlin: Springer 1998;151-155
-
- Kahn T, Bettag M, Ulrich F, et al. MRI-guided laser-induced interstitial thermotherapy of cerebral neoplasms. J Comput Assist Tomogr 1994;18:519-532 - PubMed
-
- Heimburger RF. Ultrasound augmentation of central nervous system tumor therapy. Indiana Med 1985;78:469-476 - PubMed
-
- Cline HE, Schenck JF, Hynynen K, Watkins RD, Souza SP, Jolesz FA. MR-guided focused ultrasound surgery. J Comput Assist Tomogr 1992;16:956-965 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
