Early-onset combined methylmalonic aciduria and homocystinuria: neuroradiologic findings
- PMID: 11237984
- PMCID: PMC7976836
Early-onset combined methylmalonic aciduria and homocystinuria: neuroradiologic findings
Abstract
Background and purpose: Combined methylmalonic aciduria and homocystinuria (MMA-HC) is caused by impaired hepatic conversion of dietary cobalamin to methylcobalamin and adenosylcobalamin, resulting in decreased activity of methylmalonyl-CoA mutase and methionine synthase. Patients with the early-onset variety present within 12 months of age with severe neurologic, hematologic, and gastrointestinal abnormalities. We describe the neuroradiologic features of early-onset MMA-HC and discuss related pathophysiological mechanisms.
Methods: Twelve infants with hypotonia, failure to thrive, poor feeding, and hematologic abnormalities were diagnosed with MMA-HC on the basis of a typical plasmatic and urinary metabolic profile and enzyme activity in fibroblastic cultures. Complementation studies were performed in two cases, and yielded a CblC result. MR imaging was performed at presentation in four cases and later in the others. All patients showed prompt biochemical improvement with intramuscular hydroxocobalamin administration, and most had moderate neurologic improvement.
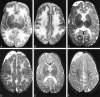
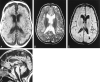
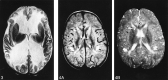
Results: Diffuse supratentorial white matter edema and dysmyelination was the typical MR picture at presentation, whereas white matter bulk loss characterized later stages of the disease. Nucleocapsular areas of gliosis were an additional finding in one case. One patient had tetraventricular hydrocephalus at presentation.
Conclusion: White matter damage is probably caused by reduced methyl group availability and nonphysiological fatty acids toxicity, whereas focal gliosis results from homocysteine-induced toxicity to the endothelium. Hydrocephalus may result from diffuse intracranial extracerebral arterial stiffness, known as reduced arterial pulsation hydrocephalus. MR imaging features at presentation and at follow-up are nonspecific.
Figures


References
-
- Baumgartner ER, Wick H, Maurer R, Egli N, Steinmann B. Congenital defect in intracellular cobalamin metabolism resulting in homocystinuria and methylmalonic aciduria. Helv Paediat Acta 1979;34:465-482 - PubMed
-
- Rosenblatt DS, Aspler AL, Shevell MI, Pletcher BA, Fenton WA, Seashore MR. Clinical heterogeneity and prognosis in combined methylmalonic aciduria and homocystinuria (cblC). J Inherit Metab Dis 1997;20:528-538 - PubMed
-
- Merinero B, Perez-Cerda C, Garcia MJ, et al. Reliability of biochemical parameters used in prenatal diagnosis of combined methylmalonic aciduria and homocystinuria. Prenat Diagn 1998;18:947-952 - PubMed
-
- Enns GM, Barkovich AJ, Rosenblatt DS, et al. Progressive neurological deterioration and MRI changes in cblC methylmalonic acidemia treated with hydroxocobalamin. J Inherit Metab Dis 1999;22:599-607 - PubMed
-
- Landis R, Koch G. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-174 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
