The beta-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors
- PMID: 11238036
- PMCID: PMC1850343
- DOI: 10.1016/s0002-9440(10)64035-7
The beta-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors
Abstract
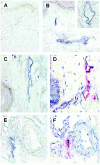
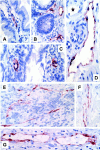
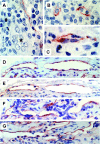
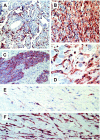
The lymphatic vessels (lymphatics) play an important role in channeling fluid and leukocytes from the tissues to the secondary lymphoid organs. In addition to driving leukocyte egress from blood, chemokines have been suggested to contribute to leukocyte recirculation via the lymphatics. Previously, we have demonstrated that binding sites for several pro-inflammatory beta-chemokines are found on the endothelial cells (ECs) of lymphatics in human dermis. Here, using the MIP-1alpha isoform MIP-1alphaP, we have extended these studies to further support the contention that the in situ chemokine binding to afferent lymphatics exhibits specificity akin to that observed in vitro with the promiscuous beta-chemokine receptor D6. We have generated monoclonal antibodies to human D6 and showed D6 immunoreactivity on the ECs lining afferent lymphatics, confirmed as such by staining serial skin sections with antibodies against podoplanin, a known lymphatic EC marker. In parallel, in situ hybridization on skin with antisense D6 probes demonstrated the expression of D6 mRNA by lymphatic ECs. D6-immunoreactive lymphatics were also abundant in mucosa and submucosa of small and large intestine and appendix, but not observed in several other organs tested. In lymph nodes, D6 immunoreactivity was present on the afferent lymphatics and also in subcapsular and medullary sinuses. Tonsilar lymphatic sinuses were also D6-positive. Peripheral blood cells and the ECs of blood vessels and high endothelial venules were consistently nonreactive with anti-D6 antibodies. Additionally, we have demonstrated that D6 immunoreactivity is detectable in some malignant vascular tumors suggesting they may be derived from, or phenotypically similar to, lymphatic ECs. This is the first demonstration of chemokine receptor expression by lymphatic ECs, and suggests that D6 may influence the chemokine-driven recirculation of leukocytes through the lymphatics and modify the putative chemokine effects on the development and growth of vascular tumors.
Figures


References
-
- Jussila L, Valtola R, Partanen TA, Salven P, Heikkila P, Matikainen M-T, Renkonen R, Kaipainen A, Detmar M, Tschachler E, Alitalo R, Alitalo K: Lymphatic endothelium and Kaposi’s sarcoma spindle cells detected by antibodies against the vascular endothelial growth factor receptor-3. Cancer Res 1998, 58:1599-1604 - PubMed
-
- Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D: Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries. Podoplanin as a specific marker for lymphatic endothelium. Am J Pathol 1999, 154:385-394 - PMC - PubMed
-
- Ebata N, Sawa Y, Ashikaga Y, Yamaoka Y, Suzuki M, Totsuka Y, Yoshida S: Lymphatic endothelium of the human tongue expresses multiple leukocyte adhesion molecules. Tissue Cell 1999, 31:34-38 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

