Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes
- PMID: 11238037
- PMCID: PMC1850342
- DOI: 10.1016/S0002-9440(10)64036-9
Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes
Abstract
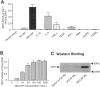
Inflammation and oxidative stress contribute to the pathogenesis of many human diseases including atherosclerosis. Advanced human atheroma contains high levels of the enzyme myeloperoxidase that produces the pro-oxidant species, hypochlorous acid (HOCl). This study documents increased numbers of myeloperoxidase-expressing macrophages in eroded or ruptured plaques causing acute coronary syndromes. In contrast, macrophages in human fatty streaks contain little or no myeloperoxidase. Granulocyte macrophage colony-stimulating factor, but not macrophage colony-stimulating factor, selectively regulates the ability of macrophages to express myeloperoxidase and produce HOCl in vitro. Moreover, myeloperoxidase-positive macrophages in plaques co-localized with granulocyte macrophage colony-stimulating factor. Pro-inflammatory stimuli known to be present in human atherosclerotic plaque, including CD40 ligand, lysophosphatidylcholine, or cholesterol crystals, could induce release of myeloperoxidase from HOCl production by macrophages in vitro. HOCl-modified proteins accumulated at ruptured or eroded sites of human coronary atheroma. These results identify granulocyte macrophage colony-stimulating factor as an endogenous regulator of macrophage myeloperoxidase expression in human atherosclerosis and support a particular role for the myeloperoxidase-expressing macrophages in atheroma complication and the acute coronary syndromes.
Figures





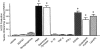
References
-
- Ross R: Atherosclerosis: an inflammatory disease. N Engl J Med 1999, 340:115-126 - PubMed
-
- Leeuwenburgh C, Rasmussen JE, Hsu FF, Mueller DM, Pennathur S, Heinecke JW: Mass spectrometric quantification of markers for protein oxidation by tyrosyl radical, copper, and hydroxyl radical in low density lipoprotein isolated from human atherosclerotic plaques. J Biol Chem 1997, 272:3520-3526 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

