Generation of C-reactive protein and complement components in atherosclerotic plaques
- PMID: 11238052
- PMCID: PMC1850354
- DOI: 10.1016/S0002-9440(10)64051-5
Generation of C-reactive protein and complement components in atherosclerotic plaques
Abstract
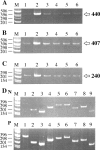
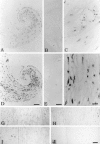
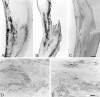
C-reactive protein (CRP) and complement are hypothesized to be major mediators of inflammation in atherosclerotic plaques. We used the reverse transcriptase-polymerase chain reaction technique to detect the mRNAs for CRP and the classical complement components C1 to C9 in both normal arterial and plaque tissue, establishing that they can be endogenously generated by arteries. When the CRP mRNA levels of plaque tissue, normal artery, and liver were compared in the same cases, plaque levels were 10.2-fold higher than normal artery and 7.2-fold higher than liver. By Western blotting, we showed that the protein levels of CRP and complement proteins were also up-regulated in plaque tissue and that there was full activation of the classical complement pathway. By in situ hybridization, we detected intense signals for CRP and C4 mRNAs in smooth muscle-like cells and macrophages in the thickened intima of plaques. By immunohistochemistry we showed co-localization of CRP and the membrane attack complex of complement. We also detected up-regulation in plaque tissue of the mRNAs for the macrophage markers CD11b and HLA-DR, as well as their protein products. We showed by immunohistochemistry macrophage infiltration of plaque tissue. Because CRP is a complement activator, and activated complement attacks cells in plaque tissue, these data provide evidence of a self-sustaining autotoxic mechanism operating within the plaques as a precursor to thrombotic events.
Figures



References
-
- Ross R: Atherosclerosis: an inflammatory disease. N Engl J Med 1999, 340:115-126 - PubMed
-
- Constantinides P: General Pathobiology. 1994:pp 59-116 Appleton & Lange, Norwalk
-
- Seifert PS, Kazatchkine MD: The complement system in atherosclerosis. Atherosclerosis 1988, 73:91-104 - PubMed
-
- Torzewski J, Bowyer DE, Waltenberger J, Fitzsimmons C: Processes in atherogenesis: complement activation. Atherosclerosis 1997, 132:131-138 - PubMed
-
- Libby P, Egan D, Skarkatos S: Roles of infectious agents in atherosclerosis and restenosis: an assessment of the evidence and need for future research. Circulation 1997, 96:4095-4103 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

