Pneumococcal type 22f polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay
- PMID: 11238206
- PMCID: PMC96047
- DOI: 10.1128/CDLI.8.2.266-272.2001
Pneumococcal type 22f polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay
Abstract
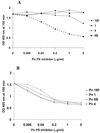
The specificity of the immune response to the 23-valent pneumococcal-polysaccharide (PS) vaccine in healthy adults and to a pneumococcal conjugate vaccine in infants was examined by measuring immunoglobulin G (IgG) antibody titers by enzyme-linked immunosorbent assay (ELISA) and the opsonophagocytosis assay. ELISA measures total antipneumococcal IgG titers including the titers of functional and nonfunctional antibodies, while the opsonophagocytosis assay measures only functional-antibody titers. Twenty-four pairs of pre- and post-pneumococcal vaccination sera from adults were evaluated (ELISA) for levels of IgG antibodies against serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F. Twelve of the pairs were also examined (opsonophagocytosis assay) for their functional activities. The correlation coefficients between assay results for most types ranged from 0.75 to 0.90, but the correlation coefficient was only about 0.6 for serotypes 4 and 19F. The specificities of these antibodies were further examined by the use of competitive ELISA inhibition. A number of heterologous polysaccharides (types 11A, 12F, 15B, 22F, and 33A) were used as inhibitors. Most of the sera tested showed cross-reacting antibodies, in addition to those removed by pneumococcal C PS absorption. Our data suggest the presence of a common epitope that is found on most pneumococcal PS but that is not absorbed by purified C PS. Use of a heterologous pneumococcal PS (22F) to adsorb the antibodies to the common epitope increased the correlation between the IgG ELISA results and the opsonophagocytosis assay results. The correlation coefficient improve from 0.66 to 0.92 for type 4 and from 0.63 to 0.80 for type 19F. These common-epitope antibodies were largely absent in infants at 7 months of age, suggesting the carbohydrate nature of the epitope.
Figures


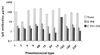
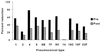

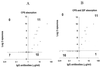

Similar articles
-
A 22-plex chemiluminescent microarray for pneumococcal antibodies.Am J Clin Pathol. 2007 Jul;128(1):23-31. doi: 10.1309/781K5W6QH7JH2TMA. Am J Clin Pathol. 2007. PMID: 17580269
-
Validation of a routine opsonophagocytosis assay to predict invasive pneumococcal disease efficacy of conjugate vaccine in children.Vaccine. 2007 Mar 22;25(13):2518-27. doi: 10.1016/j.vaccine.2006.09.029. Epub 2006 Sep 20. Vaccine. 2007. PMID: 17034907
-
[An immunoenzymatic test for IgG antibody levels against 10 serotypes of Streptococcus pneumoniae].Biomedica. 2012 Jan-Mar;32(1):92-102. doi: 10.1590/S0120-41572012000100011. Biomedica. 2012. PMID: 23235791 Spanish.
-
Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides.Clin Diagn Lab Immunol. 2003 Jul;10(4):514-9. doi: 10.1128/cdli.10.4.514-519.2003. Clin Diagn Lab Immunol. 2003. PMID: 12853378 Free PMC article. Review. No abstract available.
-
Measuring immune responses to pneumococcal vaccines.J Immunol Methods. 2018 Oct;461:37-43. doi: 10.1016/j.jim.2018.08.002. Epub 2018 Aug 8. J Immunol Methods. 2018. PMID: 30098317 Free PMC article. Review.
Cited by
-
Immunogenicity and safety of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) co-administered with DTPa vaccine in Japanese children: A randomized, controlled study.Hum Vaccin Immunother. 2015;11(4):826-37. doi: 10.1080/21645515.2015.1012019. Hum Vaccin Immunother. 2015. PMID: 25830489 Free PMC article. Clinical Trial.
-
Outpacing the pneumococcus: Antibody dynamics in the first few days following pneumococcal capsular antigen stimulation.Sci Rep. 2018 Oct 18;8(1):15376. doi: 10.1038/s41598-018-33735-x. Sci Rep. 2018. PMID: 30337597 Free PMC article. Clinical Trial.
-
Comparative immune responses of patients with chronic pulmonary diseases during the 2-year period after pneumococcal vaccination.Clin Vaccine Immunol. 2007 Feb;14(2):139-45. doi: 10.1128/CVI.00336-06. Epub 2006 Dec 13. Clin Vaccine Immunol. 2007. PMID: 17167035 Free PMC article.
-
Assignment of weight-based immunoglobulin G1 (IgG1) and IgG2 units in antipneumococcal reference serum lot 89-S(F) for pneumococcal polysaccharide serotypes 1, 4, 5, 7F, 9V, and 18C.Clin Diagn Lab Immunol. 2005 Jan;12(1):218-23. doi: 10.1128/CDLI.12.1.218-223.2005. Clin Diagn Lab Immunol. 2005. PMID: 15643011 Free PMC article.
-
Interlaboratory comparison of three multiplexed bead-based immunoassays for measuring serum antibodies to pneumococcal polysaccharides.Clin Vaccine Immunol. 2010 May;17(5):862-9. doi: 10.1128/CVI.00022-10. Epub 2010 Mar 24. Clin Vaccine Immunol. 2010. PMID: 20335434 Free PMC article.
References
-
- Anttila M, Eskola J, Åhman H, Käyhty H. Differences in the avidity of antibodies evoked by four different pneumococcal conjugate vaccines in early childhood. Vaccine. 1999;17:1970–1977. - PubMed
-
- Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen J R, Elvin L, Ensor K M, Hackell J, Siber G, Malinoski F, Madore D, Chang I, Kohberger R, Watson W, Austrian R, Edwards K Northern California Kaiser Permanente Vaccine Study Center Group. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis J. 2000;19:187–195. - PubMed
-
- Deng L Y, Kasper D L, Krick T P, Wessels M R. Characterization of the linkage between the type III capsular polysaccharide and the bacterial cell wall of group B Streptococcus. J Biol Chem. 2000;275:7497–7504. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

