A multistep damage recognition mechanism for global genomic nucleotide excision repair
- PMID: 11238373
- PMCID: PMC312644
- DOI: 10.1101/gad.866301
A multistep damage recognition mechanism for global genomic nucleotide excision repair
Abstract
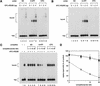
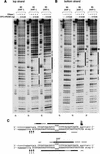
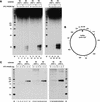
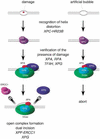
A mammalian nucleotide excision repair (NER) factor, the XPC-HR23B complex, can specifically bind to certain DNA lesions and initiate the cell-free repair reaction. Here we describe a detailed analysis of its binding specificity using various DNA substrates, each containing a single defined lesion. A highly sensitive gel mobility shift assay revealed that XPC-HR23B specifically binds a small bubble structure with or without damaged bases, whereas dual incision takes place only when damage is present in the bubble. This is evidence that damage recognition for NER is accomplished through at least two steps; XPC-HR23B first binds to a site that has a DNA helix distortion, and then the presence of injured bases is verified prior to dual incision. Cyclobutane pyrimidine dimers (CPDs) were hardly recognized by XPC-HR23B, suggesting that additional factors may be required for CPD recognition. Although the presence of mismatched bases opposite a CPD potentiated XPC-HR23B binding, probably due to enhancement of the helix distortion, cell-free excision of such compound lesions was much more efficient than expected from the observed affinity for XPC-HR23B. This also suggests that additional factors and steps are required for the recognition of some types of lesions. A multistep mechanism of this sort may provide a molecular basis for ensuring the high level of damage discrimination that is required for global genomic NER.
Figures



References
-
- Asahina H, Kuraoka I, Shirakawa M, Morita EH, Miura N, Miyamoto I, Ohtsuka E, Okada Y, Tanaka K. The XPA protein is a zinc metalloprotein with an ability to recognize various kinds of DNA damage. Mutat Res. 1994;315:229–237. - PubMed
-
- Batty D, Rapic'-Otrin V, Levine AS, Wood RD. Stable binding of human XPC complex to irradiated DNA confers strong discrimination for damaged sites. J Mol Biol. 2000;300:275–290. - PubMed
-
- Bohr VA, Smith CA, Okumoto DS, Hanawalt PC. DNA repair in active gene: Removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985;40:359–369. - PubMed
-
- Bootsma D, Kraemer KH, Cleaver J, Hoeijmakers JHJ. Nucleotide excision repair syndromes: Xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic basis of inherited disease. New York, NY: McGraw-Hill Book Co.; 1997.
-
- Burns J, Guzder S, Sung P, Prakash S, Prakash L. An affinity of human replication protein A for ultraviolet-damaged DNA. J Biol Chem. 1996;271:11607–11610. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
