Robertson's Mutator transposons in A. thaliana are regulated by the chromatin-remodeling gene Decrease in DNA Methylation (DDM1)
- PMID: 11238379
- PMCID: PMC312647
- DOI: 10.1101/gad.193701
Robertson's Mutator transposons in A. thaliana are regulated by the chromatin-remodeling gene Decrease in DNA Methylation (DDM1)
Abstract
Robertson's Mutator transposable elements in maize undergo cycles of activity and then inactivity that correlate with changes in cytosine methylation. Mutator-like elements are present in the Arabidopsis genome but are heavily methylated and inactive. These elements become demethylated and active in the chromatin-remodeling mutant ddm1 (Decrease in DNA Methylation), which leads to loss of heterochromatic DNA methylation. Thus, DNA transposons in plants appear to be regulated by chromatin remodeling. In inbred ddm1 strains, transposed elements may account, in part, for mutant phenotypes unlinked to ddm1. Gene silencing and paramutation are also regulated by DDM1, providing support for the proposition that epigenetic silencing is related to transposon regulation.
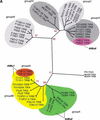
Figures





References
-
- Banks JA, Masson P, Fedoroff N. Molecular mechanisms in the developmental regulation of the maize Suppressor-mutator transposable element. Genes & Dev. 1988;2:1364–1380. - PubMed
-
- Bender J, Fink GR. Epigenetic control of an endogenous gene family is revealed by a novel blue fluorescent mutant of Arabidopsis. Cell. 1995;83:725–734. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
