Caveolin-2 is targeted to lipid droplets, a new "membrane domain" in the cell
- PMID: 11238462
- PMCID: PMC2198803
- DOI: 10.1083/jcb.152.5.1079
Caveolin-2 is targeted to lipid droplets, a new "membrane domain" in the cell
Abstract
Caveolin-1 and -2 constitute a framework of caveolae in nonmuscle cells. In the present study, we showed that caveolin-2, especially its beta isoform, is targeted to the surface of lipid droplets (LD) by immunofluorescence and immunoelectron microscopy, and by subcellular fractionation. Brefeldin A treatment induced further accumulation of caveolin-2 along with caveolin-1 in LD. Analysis of mouse caveolin-2 deletion mutants revealed that the central hydrophobic domain (residues 87-119) and the NH(2)-terminal (residues 70-86) and COOH-terminal (residues 120-150) hydrophilic domains are all necessary for the localization in LD. The NH(2)- and COOH-terminal domains appeared to be related to membrane binding and exit from ER, respectively, implying that caveolin-2 is synthesized and transported to LD as a membrane protein. In conjunction with recent findings that LD contain unesterified cholesterol and raft proteins, the result implies that the LD surface may function as a membrane domain. It also suggests that LD is related to trafficking of lipid molecules mediated by caveolins.
Figures


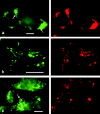

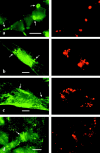



Comment in
-
Caveolin, cholesterol, and lipid droplets?J Cell Biol. 2001 Mar 5;152(5):F29-34. doi: 10.1083/jcb.152.5.f29. J Cell Biol. 2001. PMID: 11238468 Free PMC article. No abstract available.
References
-
- Anderson R.G.W. The caveolae membrane system. Annu. Rev. Biochem. 1998;67:199–225. - PubMed
-
- Blanchette-Mackie E.J., Dwyer N.K., Barber T., Coxey R.A., Takeda T., Rondinone C.M., Theodorakis J.L., Greenberg A.S., Londos C. Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J. Lipid Res. 1995;36:1211–1226. - PubMed
-
- Brasaemle D.L., Barber T., Kimmel A.R., Londos C. Post-translational regulation of perilipin expressionstabilization by stored intracellular neutral lipids. J. Biol. Chem. 1997;272:9378–9387. - PubMed
-
- Das K., Lewis R.Y., Scherer P.E., Lisanti M.P. The membrane-spanning domains of caveolins-1 and -2 mediate the formation of caveolin hetero-oligomers. Implications for the assembly of caveolae membranes in vivo. J. Biol. Chem. 1999;274:18721–18728. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

