Mammalian sprouty-1 and -2 are membrane-anchored phosphoprotein inhibitors of growth factor signaling in endothelial cells
- PMID: 11238463
- PMCID: PMC2198812
- DOI: 10.1083/jcb.152.5.1087
Mammalian sprouty-1 and -2 are membrane-anchored phosphoprotein inhibitors of growth factor signaling in endothelial cells
Abstract
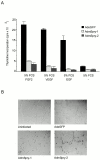
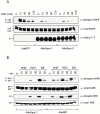
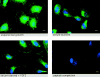
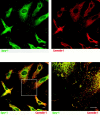
Growth factor-induced signaling by receptor tyrosine kinases (RTKs) plays a central role in embryonic development and in pathogenesis and, hence, is tightly controlled by several regulatory proteins. Recently, Sprouty, an inhibitor of Drosophila development-associated RTK signaling, has been discovered. Subsequently, four mammalian Sprouty homologues (Spry-1-4) have been identified. Here, we report the functional characterization of two of them, Spry-1 and -2, in endothelial cells. Overexpressed Spry-1 and -2 inhibit fibroblast growth factor- and vascular endothelial growth factor-induced proliferation and differentiation by repressing pathways leading to p42/44 mitogen-activating protein (MAP) kinase activation. In contrast, although epidermal growth factor-induced proliferation of endothelial cells was also inhibited by Spry-1 and -2, activation of p42/44 MAP kinase was not affected. Biochemical and immunofluorescence analysis of endogenous and overexpressed Spry-1 and -2 reveal that both Spry-1 and -2 are anchored to membranes by palmitoylation and associate with caveolin-1 in perinuclear and vesicular structures. They are phosphorylated on serine residues and, upon growth factor stimulation, a subset is recruited to the leading edge of the plasma membrane. The data indicate that mammalian Spry-1 and -2 are membrane-anchored proteins that negatively regulate angiogenesis-associated RTK signaling, possibly in a RTK-specific fashion.
Figures







References
-
- Anderson R.G. The caveolae membrane system. Annu. Rev. Biochem. 1998;67:199–225. - PubMed
-
- Baatout S. Endothelial differentiation using Matrigel. Anticancer Res. 1997;17:451–455. - PubMed
-
- Baker A., Saltik M., Lehrman H., Killisch I., Mautner V., Lamm G., Christofori G., Cotten M. Polyethylenimine (PEI) is a simple, inexpensive and effective reagent for condensing and linking plasmid DNA to adenovirus for gene delivery. Gene Therapy. 1997;4:773–782. - PubMed
-
- Casci T., Vinos J., Freeman M. Sprouty, an intracellular inhibitor of Ras signalling. Cell. 1999;96:655–665. - PubMed
-
- Chambers D., Mason I. Expression of sprouty2 during early development of the chick embryo is coincident with known sites of FGF signalling. Mech. Dev. 2000;91:361–364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

