Hepatic cholesterol metabolism and resistance to dietary cholesterol in LXRbeta-deficient mice
- PMID: 11238557
- PMCID: PMC199420
- DOI: 10.1172/JCI9794
Hepatic cholesterol metabolism and resistance to dietary cholesterol in LXRbeta-deficient mice
Abstract
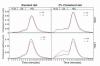
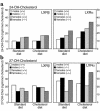
The nuclear oxysterol-receptor paralogues LXRalpha and LXRbeta share a high degree of amino acid identity and bind endogenous oxysterol ligands with similar affinities. While LXRalpha has been established as an important regulator of cholesterol catabolism in cholesterol-fed mice, little is known about the function of LXRbeta in vivo. We have generated mouse lines with targeted disruptions of each of these LXR receptors and have compared their responses to dietary cholesterol. Serum and hepatic cholesterol levels and lipoprotein profiles of cholesterol-fed animals revealed no significant differences between LXRbeta(-/-) and wild-type mice. Steady-state mRNA levels of 3-hydroxy-3-methylglutaryl coenzyme A reductase, farnesyl diphosphate synthase, and squalene synthase were increased in LXRbeta(-/-) mice compared with LXRbeta(+/+) mice, when fed standard chow. The mRNA levels for cholesterol 7alpha-hydroxylase, oxysterol 7alpha-hydroxylase, sterol 12alpha-hydroxylase, and sterol 27-hydroxylase, respectively, were comparable in these strains, both on standard and 2% cholesterol chow. Our results indicate that LXRbeta(-/-) mice - in contrast to LXRalpha(-/-) mice - maintain their resistance to dietary cholesterol, despite subtle effects on the expression of genes coding for enzymes involved in lipid metabolism. Thus, our data indicate that LXRbeta has no complete overlapping function compared with LXRalpha in the liver.
Figures




References
-
- Gordon T, Kannel WB, Castelli WP, Dawber TR. Lipoproteins, cardiovascular disease, and death. The Framingham study. Arch Intern Med. 1981;141:1128–1131. - PubMed
-
- Keys A. Coronary heart disease, serum cholesterol, and the diet. Acta Med Scand. 1980;207:153–160. - PubMed
-
- Angelin B. 1994 Mack-Forster Award Lecture. Review. Studies on the regulation of hepatic cholesterol metabolism in humans. Eur J Clin Invest. 1995;25:215–224. - PubMed
-
- Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. - PubMed
-
- Accad M, Farese RV., Jr Cholesterol homeostasis: a role for oxysterols. Curr Biol. 1998;8:R601–R604. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

