Identification of a crucial energetic footprint on the alpha1 helix of human histocompatibility leukocyte antigen (HLA)-A2 that provides functional interactions for recognition by tax peptide/HLA-A2-specific T cell receptors
- PMID: 11238586
- PMCID: PMC2193388
- DOI: 10.1084/jem.193.5.551
Identification of a crucial energetic footprint on the alpha1 helix of human histocompatibility leukocyte antigen (HLA)-A2 that provides functional interactions for recognition by tax peptide/HLA-A2-specific T cell receptors
Abstract
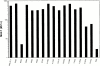
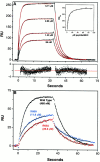
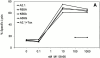
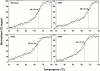
Structural studies have shown that class I major histocompatibility complex (MHC)-restricted peptide-specific T cell receptor (TCR)-alpha/betas make multiple contacts with the alpha1 and alpha2 helices of the MHC, but it is unclear which or how many of these interactions contribute to functional binding. We have addressed this question by performing single amino acid mutagenesis of the 15 TCR contact sites on the human histocompatibility leukocyte antigen (HLA)-A2 molecule recognized by the A6 TCR specific for the Tax peptide presented by HLA-A2. The results demonstrate that mutagenesis of only three amino acids (R65, K66, and A69) that are clustered on the alpha1 helix affected T cell recognition of the Tax/HLA-A2 complex. At least one of these three mutants affected T cell recognition by every member of a large panel of Tax/HLA-A2-specific T cell lines. Biacore measurements showed that these three HLA-A2 mutations also altered A6 TCR binding kinetics, reducing binding affinity. These results show that for Tax/HLA-A2-specific TCRs, there is a location on the central portion of the alpha1 helix that provides interactions crucial to their function with the MHC molecule.
Figures








References
-
- Garcia K., Degano M., Stanfield R., Brunmark A., Jackson M., Peterson P., Teyton L., Wilson I. An αβ T cell receptor structure at 2.5 Å and its orientation in the TCR-MHC complex. Science. 1996;274:209–219. - PubMed
-
- Garboczi D., Ghosh P., Utz U., Fan Q., Biddison W., Wiley D.C. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature. 1996;384:134–141. - PubMed
-
- Garcia K., Degano M., Pease L., Huang M., Peterson P., Teyton L., Wilson I. Structural basis of plasticity in T cell receptor recognition of a self peptide-MHC antigen. Science. 1998;279:1166–1172. - PubMed
-
- Ding Y.-H., Smith K., Garboczi D., Utz U., Biddison W., Wiley D.C. Two human T cell receptors bind in a similar diagonal mode to the HLA- A2/Tax peptide complex using different TCR amino acids. Immunity. 1998;8:403–411. - PubMed
-
- Ding Y.-H., Baker B.M., Garboczi D., Biddison W., Wiley D.C. Four A6-TCR/peptide/HLA-A2 structures that generate very different T cell signals are nearly identical. Immunity. 1999;11:45–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

