Aberrant in vivo T helper type 2 cell response and impaired eosinophil recruitment in CC chemokine receptor 8 knockout mice
- PMID: 11238588
- PMCID: PMC2193397
- DOI: 10.1084/jem.193.5.573
Aberrant in vivo T helper type 2 cell response and impaired eosinophil recruitment in CC chemokine receptor 8 knockout mice
Abstract
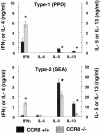
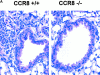
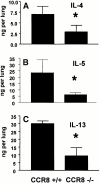
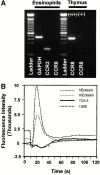
Chemokine receptors transduce signals important for the function and trafficking of leukocytes. Recently, it has been shown that CC chemokine receptor (CCR)8 is selectively expressed by Th2 subsets, but its functional relevance is unclear. To address the biological role of CCR8, we generated CCR8 deficient (-/-) mice. Here we report defective T helper type 2 (Th2) immune responses in vivo in CCR8(-/)- mice in models of Schistosoma mansoni soluble egg antigen (SEA)-induced granuloma formation as well as ovalbumin (OVA)- and cockroach antigen (CRA)-induced allergic airway inflammation. In these mice, the response to SEA, OVA, and CRA showed impaired Th2 cytokine production that was associated with aberrant type 2 inflammation displaying a 50 to 80% reduction in eosinophils. In contrast, a prototypical Th1 immune response, elicited by Mycobacteria bovis purified protein derivative (PPD) was unaffected by CCR8 deficiency. Mechanistic analyses indicated that Th2 cells developed normally and that the reduction in eosinophil recruitment was likely due to systemic reduction in interleukin 5. These results indicate an important role for CCR8 in Th2 functional responses in vivo.
Figures










References
-
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. - PubMed
-
- Strieter R.M., Polverini P.J., Arenberg D.A., Kunkel S.L. The role of CXC chemokines as regulators of angiogenesis. Shock. 1995;4:155–160. - PubMed
-
- Taub D.D., Turcovski-Corrales S.M., Key M.L., Longo D.L., Murphy W.J. Chemokines and T lymphocyte activationI. Beta chemokines costimulate human T lymphocyte activation in vitro. J. Immunol. 1996;156:2095–2103. - PubMed
-
- Taub D.D., Ortaldo J.R., Turcovski-Corrales S.M., Key M.L., Longo D.L., Murphy W.J. Beta chemokines costimulate lymphocyte cytolysis, proliferation, and lymphokine production. J. Leuk. Biol. 1996;59:81–89. - PubMed
-
- Sallusto F. The role of chemokines and chemokine receptors in T cell priming and Th1/Th2-mediated responses. Haematologica. 1999;84:28–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

