Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2
- PMID: 11238591
- PMCID: PMC2193389
- DOI: 10.1084/jem.193.5.607
Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2
Abstract
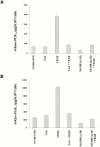
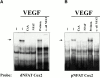
Cyclosporin A (CsA) is an immunosuppressive drug that inhibits the activity of transcription factors of the nuclear factor of activated T cells (NFAT) family, interfering with the induction of cytokines and other inducible genes required for the immune response. Here we show that CsA inhibits migration of primary endothelial cells and angiogenesis induced by vascular endothelial growth factor (VEGF); this effect appears to be mediated through the inhibition of cyclooxygenase (Cox)-2, the transcription of which is activated by VEGF in primary endothelial cells. Consistent with this, we show that the induction of Cox-2 gene expression by VEGF requires NFAT activation. Most important, the CsA-mediated inhibition of angiogenesis both in vitro and in vivo was comparable to the Cox-2 inhibitor NS-398, and reversed by prostaglandin E(2). Furthermore, the in vivo corneal angiogenesis induced by VEGF, but not by basic fibroblast growth factor, was selectively inhibited in mice treated with CsA systemically. These findings involve NFAT in the regulation of Cox-2 in endothelial cells, point to a role for this transcription factor in angiogenesis, and may provide a novel mechanism underlying the beneficial effects of CsA in angiogenesis-related diseases such as rheumatoid arthritis and psoriasis.
Figures



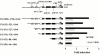










References
-
- Ferrara N., Carver-Moore K., Chen H., Dowd M., Lu L., O'Shea K.S., Powell-Braxton L., Hillan K.J., Moore M.W. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. - PubMed
-
- Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J. Mol. Med. 1999;77:527–543. - PubMed
-
- Carmeliet P., Ferreira V., Breier G., Pollefeyt S., Kieckens L., Gertsenstein M., Fahrig M., Vandenhoeck A., Harpal K., Eberhardt C. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. - PubMed
-
- Gerber H.P., Hillan K.J., Ryan A.M., Kowalski J., Keller G.A., Rangell L., Wright B.D., Radtke F., Aguet M., Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. - PubMed
-
- Ferrara N., Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997;18:4–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

